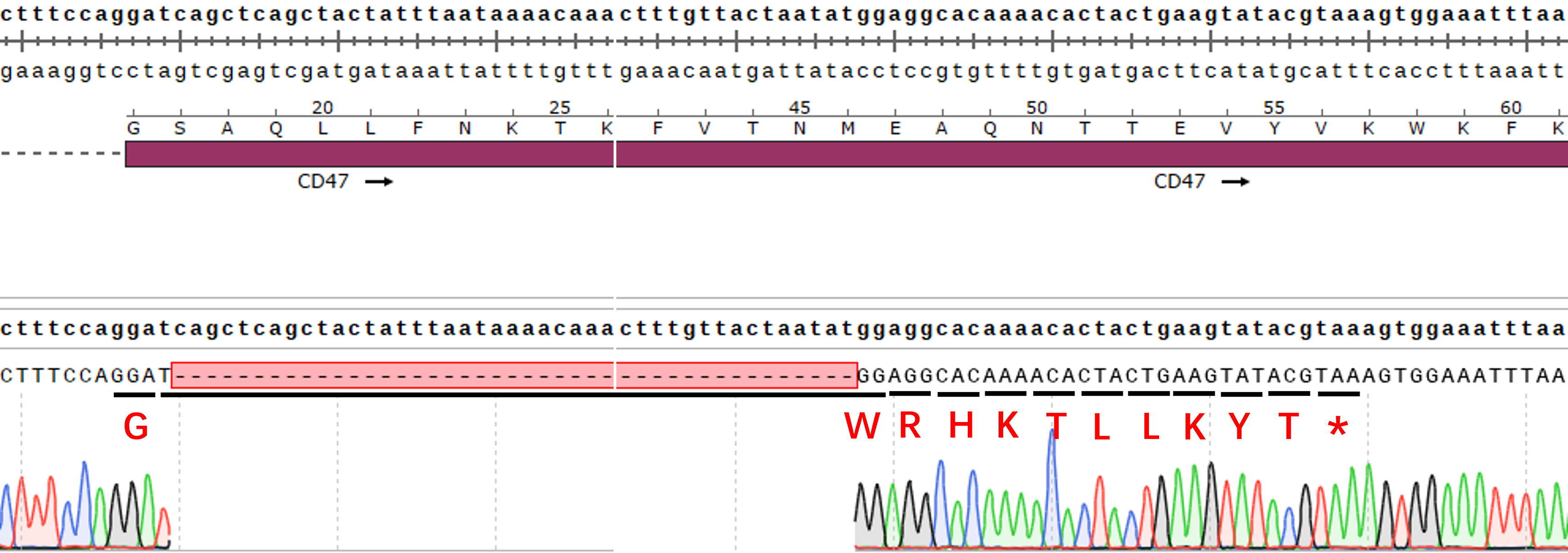
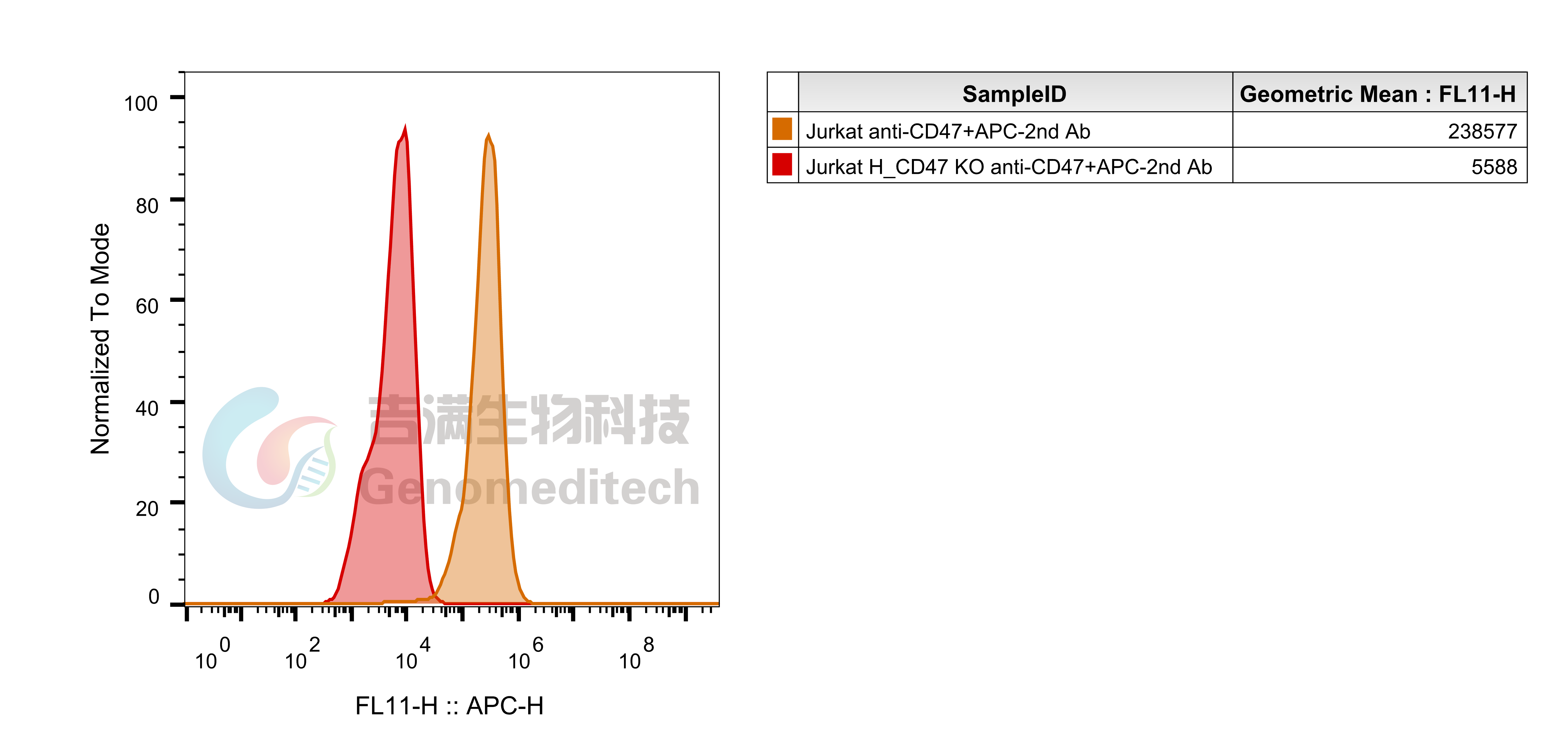
| Cat. No: GM-C28270 Product: H_SIRPα Reporter Jurkat Cell Line SIRPα (Signal Regulatory Protein Alpha) is a cell surface protein in the immunoglobulin superfamily, mainly expressed on immune cells like macrophages and dendritic cells. It regulates immune responses by binding to its ligand CD47, primarily inhibiting macrophage phagocytosis, which is essential for immune surveillance and self-tolerance. In signaling pathways, SIRPα activates downstream mechanisms through CD47 binding, inhibiting Src family tyrosine kinases. This interaction leads to the phosphorylation of SIRPα's intracellular domain, reducing macrophage activation and phagocytosis. SIRPα also affects immune cell function and tumor microenvironment formation by regulating cytokine release and intercellular interactions, making it important for research on tumor immune evasion and autoimmune diseases. H_SIRPα Reporter Jurkat Cell Line is a clonal stable Jurkat cell line constructed using lentiviral technology, constitutive expression of the human SIRPα, and exhibits signal-dependent expression of a luciferase reporter gene. The reporter cell line is co-cultured with the H_CD47 aAPC CHO-K1 Cell Line (Cat. GM-C13353). The interaction between CD47 and SIRPα inhibits TCR-CD3 signaling. By adding Anti-CD47 and Anti-SIRPα antibodies, the interactions of CD47-SIRPα are blocked, thereby restoring T cell signaling. The luciferase readout indicates the activation level of the signaling pathway, allowing evaluation of the in vitro effects of CD47-SIRPα related drugs. |  |