Cat.No:GM-87844AB
Product:Anti-FOLR1-DM4(Dar3.4)[Mirvetuximab soravtansine]
Cat.No:GM-87844AB
Product:Anti-FOLR1-DM4(Dar3.4)[Mirvetuximab soravtansine]
GM-87844AB-100 100μg
GM-87844AB-1mg 1mg
Expression System CHO
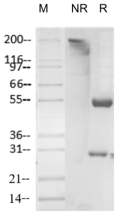
Purity >95% as determined by SDS-PAGE
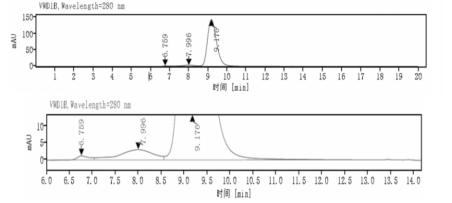
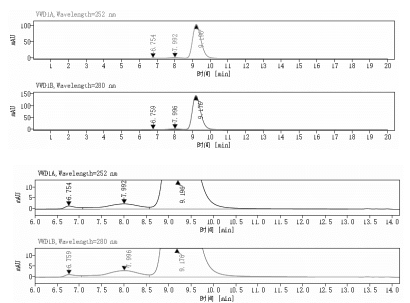
Aggregation < 5% as determined by SEC-HPLC
Drug-to-Antibody Ratio (DAR) 3.0-4.0
Endotoxin <1 EU/mg
Sterility 0.22 μm Filtered
Target FOLR1
Clone Mirvetuximab soravtansine
Alternative Names FBP, FOLR, FRalpha, NCFTD
Source/lsotype Monoclonal Human IgG1,Kappa
Application Positive control of Cytotoxicity Assay
Description Mirvetuximab soravtansine-gynx (MIRV) is a first-in-class antibody-drug conjugate targeting folate receptor α (FRα), which has been approved in the United States for the treatment of platinum-resistant ovarian cancer. Mirvetuximab soravtansine (IMGN853) is an antibody-drug conjugate (ADC) composed of the cytotoxic maytansinoid DM4, covalently linked to the humanized monoclonal antibody M9346A. Mirvetuximab soravtansine selectively binds to folate receptor 1 (FOLR1). It demonstrates antiproliferative effects by inhibiting growth and enhancing DNA damage.
Formulation Phosphate-buffered solution, pH 7.2.
Cat.No:GM-87844AB
Product:Anti-FOLR1-DM4(Dar3.4)[Mirvetuximab soravtansine]
GM-87844AB-100 100μg
GM-87844AB-1mg 1mg
Expression System CHO
Purity >95% as determined by SDS-PAGE
Aggregation < 5% as determined by SEC-HPLC
Drug-to-Antibody Ratio (DAR) 3.0-4.0
Endotoxin <1 EU/mg
Sterility 0.22 μm Filtered
Target FOLR1
Clone Mirvetuximab soravtansine
Alternative Names FBP, FOLR, FRalpha, NCFTD
Source/lsotype Monoclonal Human IgG1,Kappa
Application Positive control of Cytotoxicity Assay
Description Mirvetuximab soravtansine-gynx (MIRV) is a first-in-class antibody-drug conjugate targeting folate receptor α (FRα), which has been approved in the United States for the treatment of platinum-resistant ovarian cancer. Mirvetuximab soravtansine (IMGN853) is an antibody-drug conjugate (ADC) composed of the cytotoxic maytansinoid DM4, covalently linked to the humanized monoclonal antibody M9346A. Mirvetuximab soravtansine selectively binds to folate receptor 1 (FOLR1). It demonstrates antiproliferative effects by inhibiting growth and enhancing DNA damage.
Formulation Phosphate-buffered solution, pH 7.2.


