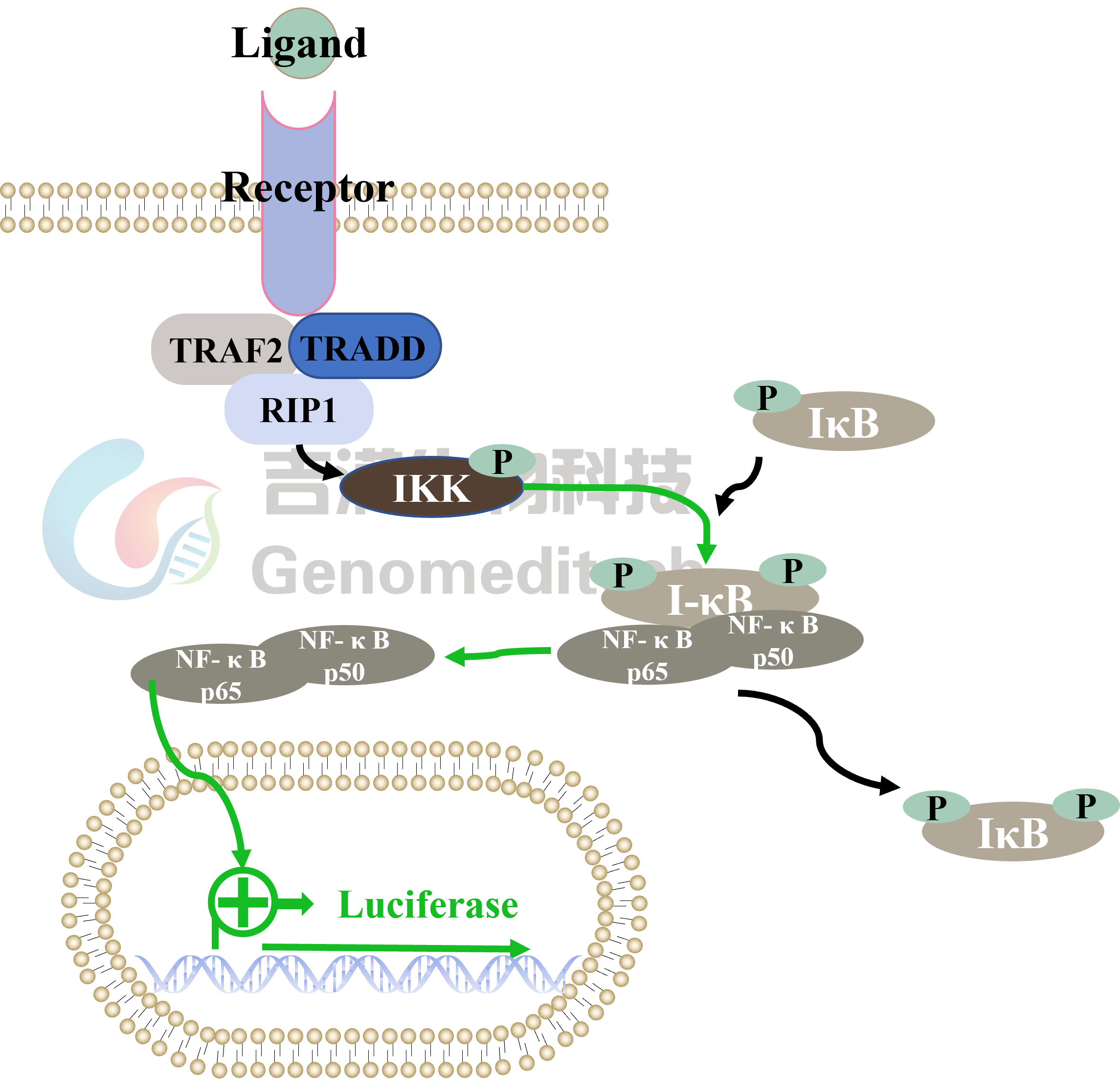
Cell Recovery
Recovery Medium: DMEM+10% FBS+1% P.S
To insure the highest level of viability, thaw the vial and initiate the culture as soon as possible upon receipt. If upon arrival, continued storage of the frozen culture is necessary, it should be stored in liquid nitrogen vapor phase and not at -70°C. Storage at -70°C will result in loss of viability.
a) Thaw the vial by gentle agitation in a 37°C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 2 - 3 minutes).
b) Remove the vial from the water bath as soon as the contents are thawed, and decontaminate by dipping in or spraying with 70% ethanol. All of the operations from this point on should be carried out under strict aseptic conditions.
c) Transfer the vial contents to a centrifuge tube containing 5.0 mL complete culture medium and spin at approximately 176 x g for 5 minutes. Discard supernatant.
d) Resuspend cell pellet with the recommended recovery medium. And dispense into appropriate culture dishes.
e) Incubate the culture at 37°C in a suitable incubator. A 5% CO₂ in air atmosphere is recommended if using the medium described on this product sheet.
Freezing Medium: 90% FBS+10%DMSO
a) Centrifuge at 176 x g for 3 minutes to collect cells.
b) Resuspend the cells in pre-cooled freezing medium and adjust the cell density to 5E6 cells/mL.
c) Aliquot 1 mL into each vial.
d) Place the vial in a controlled-rate freezing container and store at -80°C for at least 1 day, then transfer to liquid nitrogen as soon as possible.
Cell passage
Growth medium: DMEM+10% FBS+1% P.S+4 μg/mL Blasticidin
For the first 1 to 2 passages post-resuscitation, use the recovery medium. Once the cells have stabilized, switch to a growth medium.
a) Subculturing is necessary when the cell density reaches 80%. It is recommended to perform subculturing at a ratio of 1:3 to 1:4 every 2-3 days. Ensure that the density does not exceed 80%, as overcrowding can lead to reduced viability due to compression.
b) Remove and discard culture medium.
c) Briefly rinse the cell layer with PBS to remove all traces of serum that contains trypsin inhibitor.
d) Add 1.0 mL of 0.25% (w/v) Trypsin-EDTA solution to dish and observe cells under an inverted microscope until cell layer is dispersed (usually within 30 to 60 seconds at 37°C).
e) Note: To avoid clumping do not agitate the cells by hitting or shaking the flask while waiting for the cells to detach. Cells that are difficult to detach may be placed at 37°C to facilitate dispersal.
f) Add 2.0 mL of growth medium to mix well and aspirate cells by gently pipetting.
g) After centrifugation, resuspend the pellet and add appropriate aliquots of the cell suspension to new culture vessels.
h) Incubate cultures at 37°C.
Subcultivation Ratio: A subcultivation ratio of 1:3 - 1:4 is recommended
Medium Renewal: Every 2 to 3 days
Notes
a) Upon initial thawing, a higher number of dead cells is observed, which is a normal phenomenon. Significant improvement is seen after adaptation. Once the cells reach a stable state, the number of dead cells decreases after subculturing and the cell growth rate becomes stable.
b) Ensure that the cell density does not exceed 80%, as overcrowding may lead to reduced viability due to compression.