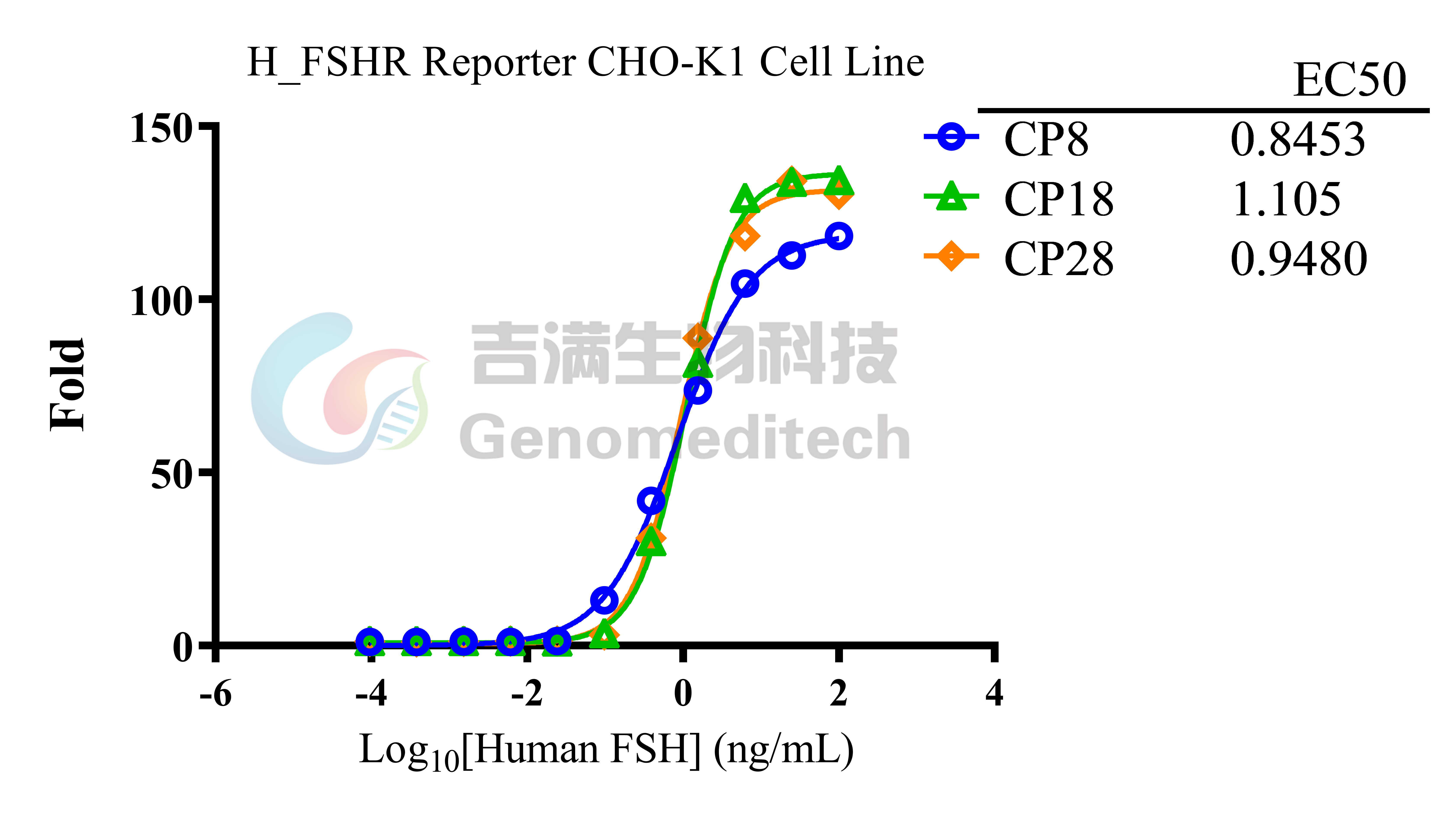
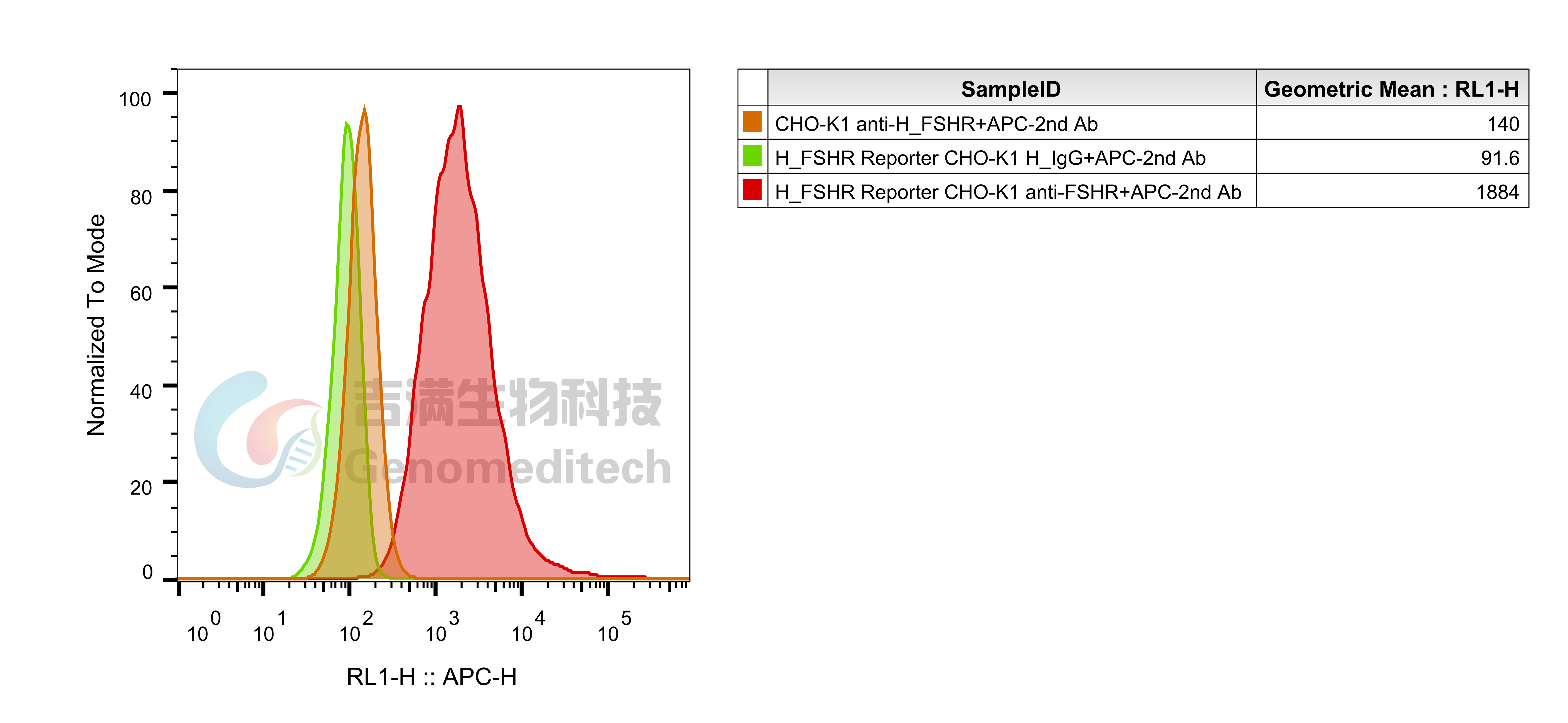
| Cat. No: GM-C25571 Product: H_FSHR Reporter CHO-K1 Cell Line FSHR (follicle-stimulating hormone receptor) is a key G protein-coupled receptor found in the reproductive cells of the ovaries and testes. It mediates the effects of follicle-stimulating hormone (FSH) and regulates reproductive system development and function. In females, FSHR activation promotes follicle growth and maturation, while in males, it aids sperm production and maturation. Abnormal FSHR expression or function can lead to infertility. The FSHR signaling pathway is primarily mediated by G proteins, especially Gαs, which activate adenylate cyclase (AC) and increase cyclic adenosine monophosphate (cAMP) levels. Elevated cAMP activates protein kinase A (PKA), regulating various downstream molecules, including transcription factors. FSHR can also activate the MAPK pathway via β-arrestin, influencing cell proliferation and survival. These signaling interactions are essential for the proper function of reproductive cells. H_FSHR Reporter CHO-K1 Cell Line is a clonal stable CHO-K1 cell line constructed using lentiviral technology, constitutive expression of the FSHR gene, along with signal-dependent expression of a luciferase reporter gene. When FSH binds to FSHR, it activates downstream signaling pathways, leading to the expression of luciferase. The luciferase activity measurement indicates the activation level of the signaling pathway and can thus be used to evaluate the in vitro effects of drugs related to FSHR. | 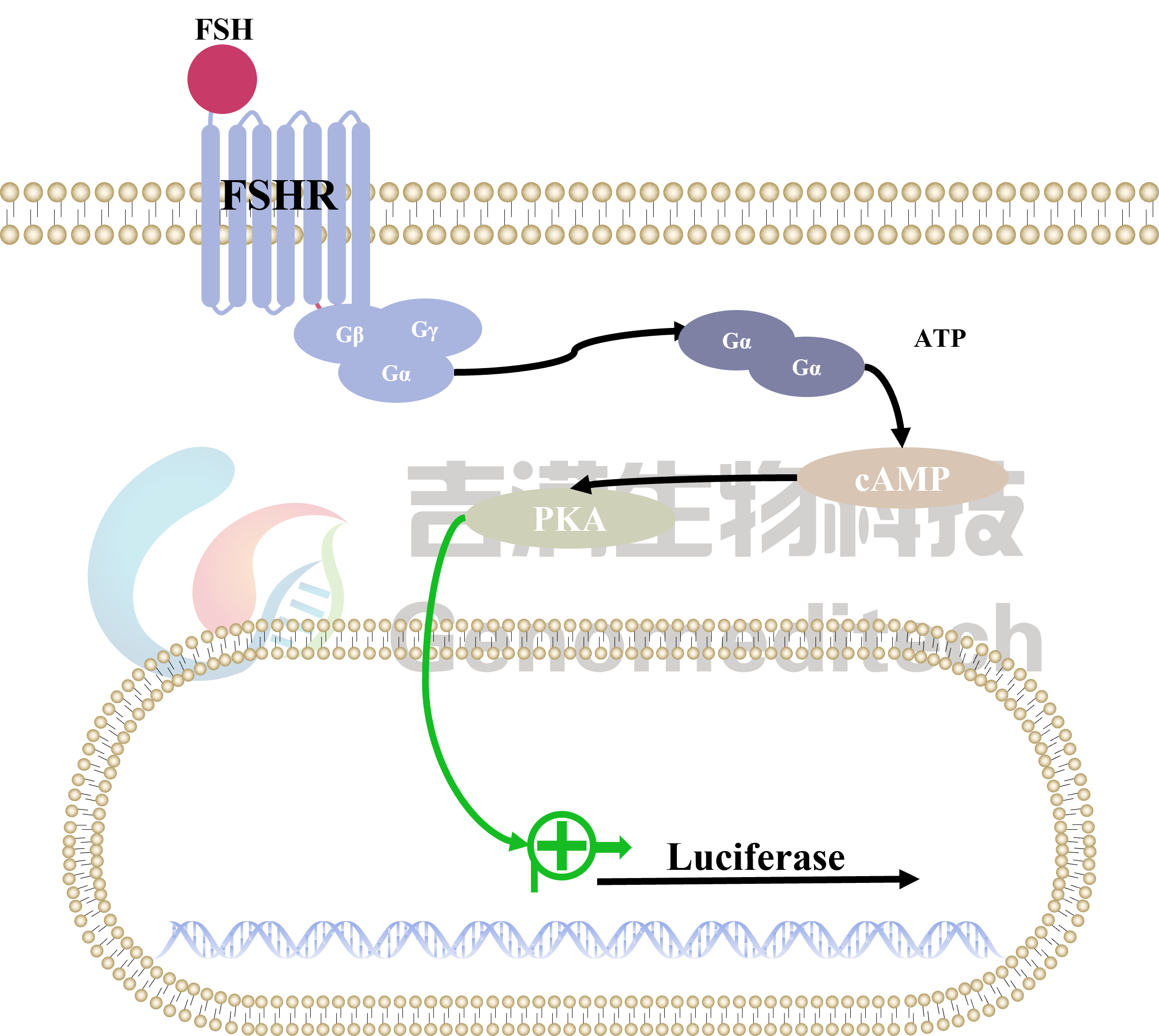 |