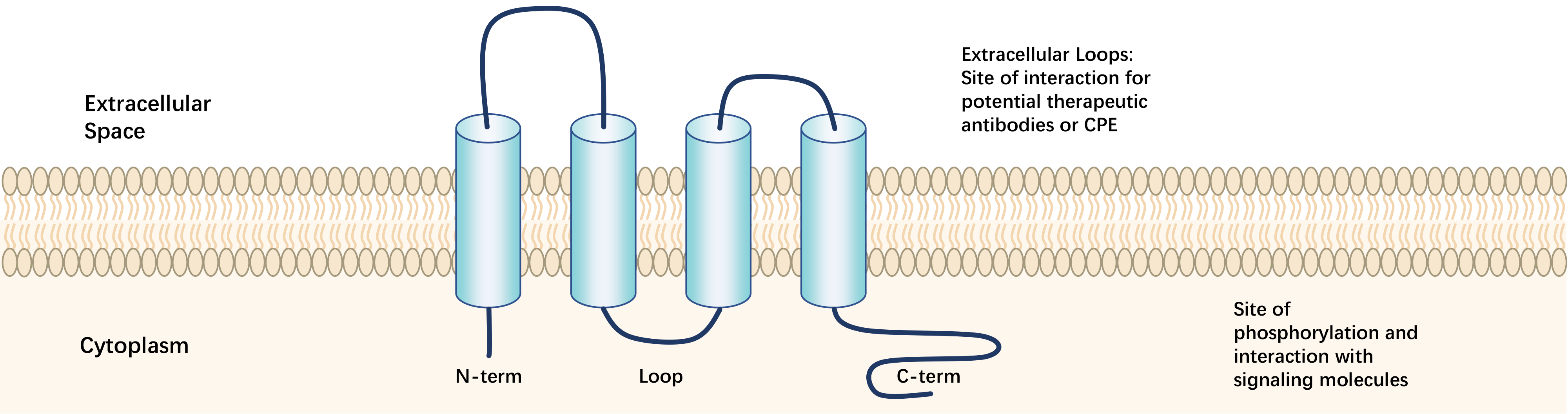
The Claudin protein family is encoded by the CLDN gene family, consisting of proteins with a relative molecular weight of 25,000-27,000. In mammals, it is composed of 27 members with high sequence homology, subject to post-translational and post-transcriptional regulation. The secondary structure of Claudin proteins consists mainly of helical bundles and β-sheets, with 4 tightly bound helical bundles forming 4 transmembrane domains TM1-TM4 embedded in the cell membrane.
Claudin proteins are predominantly expressed in epithelial cells of the body, exhibiting high tissue specificity. Different Claudin proteins show varied distribution in different organs. The tissue specificity of Claudin proteins leads to differential impacts on tumors; for instance, Claudin 5 and Claudin 9 are downregulated in cervical cancer, while Claudin 8 is upregulated. Claudin proteins play important roles in various common tumors including breast cancer, liver cancer, lung cancer, colorectal cancer, and gastric cancer.
The gastric mucosal barrier, formed by the cellular membrane of gastric epithelial cells at the top and tight connections with adjacent cells, is the main mechanism protecting the gastric mucosa. As crucial proteins related to the connection of gastric epithelial cells, abnormal expression of Claudins may compromise the gastric mucosal barrier, leading to diseases like gastric cancer. Moreover, dysfunctional tight connections resulting from abnormal Claudin protein expression can promote tumor growth and metastasis.