NK cells, typically identified in humans as CD3-CD56+ cells and in some mouse strains as CD3-NK1.1+, account for about 5-15% of lymphocytes in circulation. In addition to peripheral blood, NK cells are also found in non-lymphoid tissues such as the liver, uterus, adipose tissue, and intestines. In humans, NK cells can be classified into two subsets based on CD56 expression levels: CD56dim and CD56bright, reflecting cytotoxic and helper cell functions, respectively.
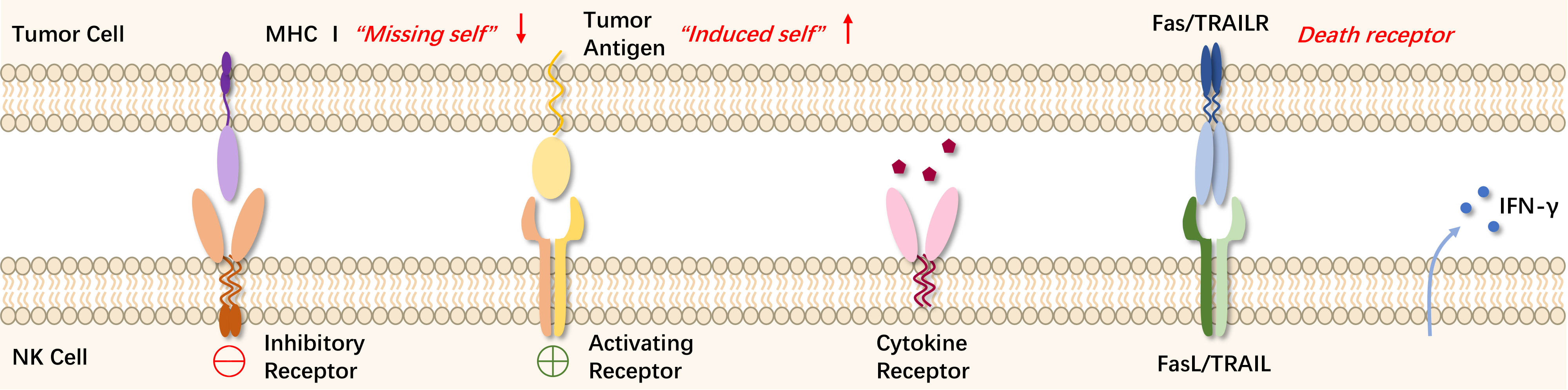
The killer cell immunoglobulin-like receptors (KIRs) expressed on the surface of NK cells can recognize major histocompatibility complex I (MHC I), which is widely expressed on normal cells to provide an inhibitory signal protecting them from NK cell attack. However, in cells undergoing viral infection or malignant transformation, MHC-I expression may be reduced or lost, allowing NK cells to escape KIR-mediated inhibition and exhibit their cytotoxic function. Apart from the "missing self" mechanism, NK cell-mediated immune surveillance also involves the "induced self." Activation receptors on NK cells include natural cytotoxicity receptors (NCR) and natural killer group 2D (NKG2D), which can recognize ligands on stressed cells and transmit activation signals. The balance between inhibitory and activating receptors determines the functional state of NK cells.