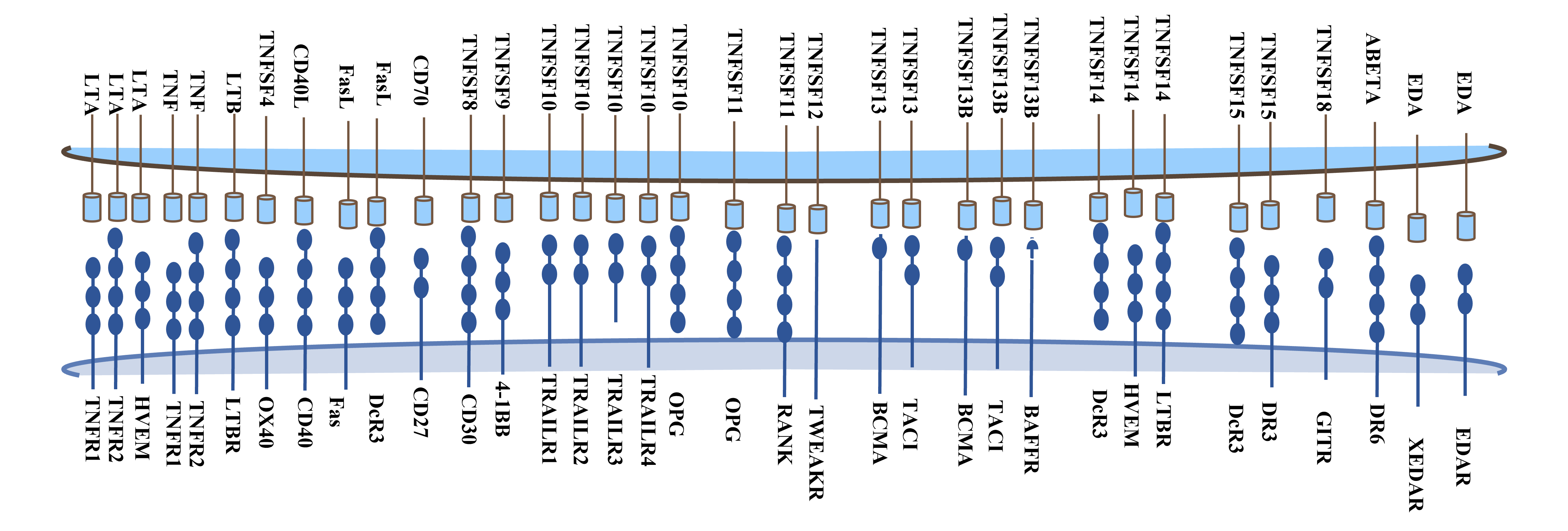
Tumor necrosis factor (TNF) was initially named for its ability to cause tumor tissue necrosis. The TNF superfamily now includes 19 ligands and 29 different receptors.
Members of the TNF superfamily ligands include TNF-α, TNF-β, lymphotoxin-β, CD40L, FasL, CD30L, 4-1BBL, CD27L, OX40L, TNF-related apoptosis-inducing ligand (TRAIL), LIGHT receptor activator of nuclear factor κB ligand (RANKL), TNF-related apoptosis-inducing ligand (TWEAK), a proliferation-inducing ligand (APRIL), B-cell activating factor (BAFF), vascular endothelial growth inhibitor (VEGI), EDA-A1 cysteine protein, EDA-A2, and GITRL. Many immune and non-immune cell types can produce TNF, including macrophages, T cells, mast cells, granulocytes, natural killer (NK) cells, and non-hematopoietic cells, participating in physiological processes such as host defense, inflammation, cell apoptosis, autoimmunity, and the development and organogenesis of the immune, ectoderm, and nervous systems.
Members of the TNF superfamily receptors (TNFR) include DR1, TNFR1, OX40, CD40, CD27, CD30, 4-1BB, BCMA, among others, mainly as transmembrane proteins. Almost all members of the TNF superfamily have pro-inflammatory activity, some activated by the transcription factor NF-kB. Some members activate various mitogen-activated protein kinases, exhibiting proliferative activity in hematopoietic cells, while others play a role in cell apoptosis.