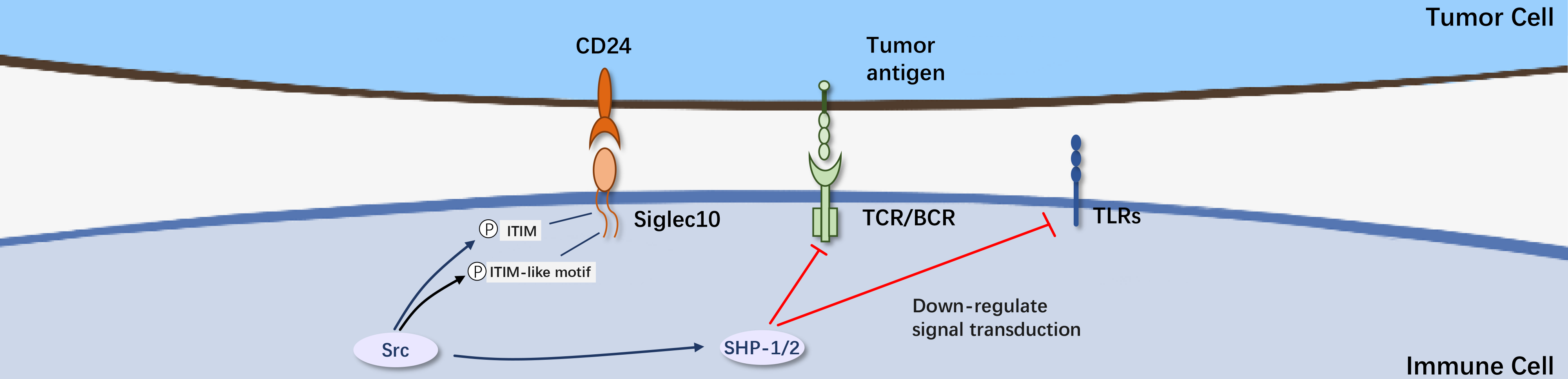
CD24, also known as heat-stable antigen, is a highly glycosylated glycosylphosphatidylinositol-anchored surface protein. It is known to interact with the Siglec-10 protein on innate immune cells, inhibiting destructive inflammatory responses related to infections, sepsis, liver damage, and chronic graft-versus-host disease. By binding to Siglec-10 on macrophages, CD24 activates an inhibitory signaling pathway mediated by SHP-1/SHP-2, releasing a "don't eat me" signal, evading immune surveillance and avoiding phagocytosis. The blockade of the CD24 signaling pathway offers a promising theoretical basis for cancer therapy, potentially treating breast and ovarian cancer. CD24, as a potential target for future immunotherapy, represents a very promising focus in developing cancer immunotherapies.
The fusion protein CD24-Fc, comprised of the extracellular portion of CD24 and the human IgG Fc fragment, triggers Siglec G signaling in mouse models, suppressing inflammatory reactions in vivo. CD24-Fc is considered a novel approach to modulating the body's inflammatory response to tissue damage, with significant implications in cancer, metabolic syndrome, and graft-versus-host disease. With the outbreak of the COVID-19 pandemic, OncoImmune initiated a Phase III clinical trial of CD24-Fc fusion protein for treating severe cases on April 20th of this year, marking a significant breakthrough as the world's first Phase III therapy for severe COVID-19. The interaction between CD24 and Siglec inhibits inflammation mediated by damage-associated molecular patterns (DAMPs), and CD24-Fc attenuates virus-induced inflammatory responses, effectively alleviating the condition of severe COVID-19 patients.