On 7 Nov, AstraZeneca announced that it will present clinical results for its next-generation T-cell splicer, AZD0486, in relapsed/refractory follicular lymphoma (R/R FL) at this year's American Society of Hematology (ASH) Annual Meeting.

AZD0486, a novel IgG4 fully human CD19xCD3 bispecific T-cell engager (TCE), is uniquely designed to bind CD3 with low affinity to reduce cytokine release upon T-cell activation, while preserving effective T-cell cytotoxicity against malignant B cells. AZD0486 was active and well tolerated in patients (pts) with relapsed/refractory (R/R) B-cell non-Hodgkin lymphoma (B-NHL), including those with R/R diffuse large B-cell lymphoma (DLBCL) (Gaballa, et al. Blood. 2023). Here, we present interim results from the ongoing phase 1, dose-escalation trial of AZD0486 in pts with R/R DLBCL (NCT04594642).
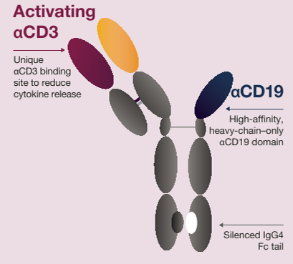
Drug structure of AZD0486














