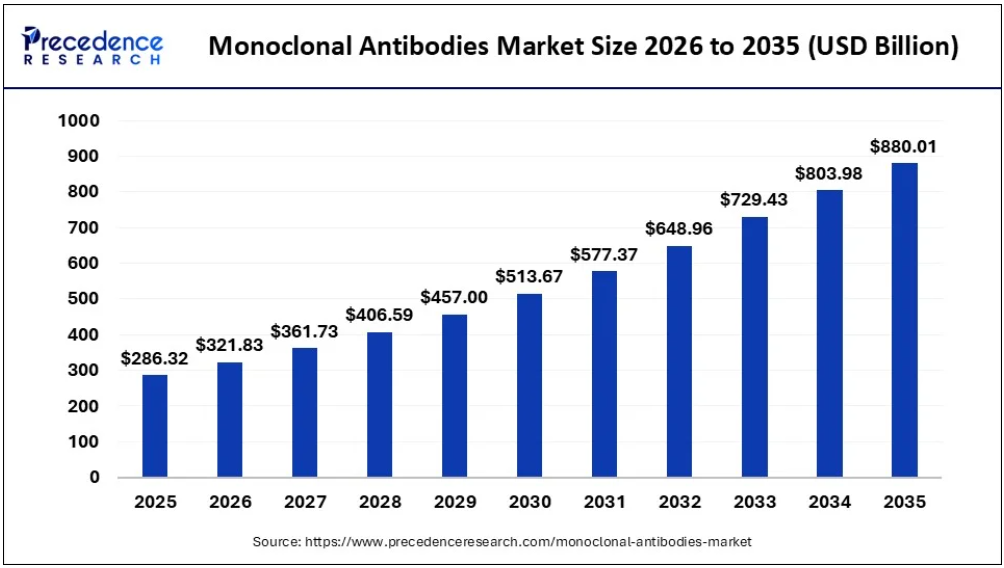
According to Precedence Research, the global monoclonal antibody market is expected to reach USD 286.32 billion in 2025 and expand from USD 321.83 billion in 2026 to approximately USD 880.01 billion by 2035. This reflects a compound annual growth rate (CAGR) of 11.88% over the period from 2026 to 2034.
With advances in antibody engineering and protein design, antibodies have evolved from natural immune molecules into highly customizable therapeutic platforms, playing an indispensable role in oncology, autoimmune disorders, and infectious diseases. Beyond antigen specificity, antibodies leverage their Fc domains to mediate multiple effector functions—including ADCC, ADCP, and CDC—enabling targeted cytotoxicity and immune modulation.
This article provides a concise overview of antibody structure, classification, and the three major effector mechanisms (ADCC, ADCP, and CDC), highlighting the fundamental functions and development value of antibody-based therapeutics.
The Meaning, Structure, and Fundamental Functions of Antibodies
Antibodies (Ab), also known as immunoglobulins (Ig), are large proteins belonging to the immunoglobulin superfamily. They recognize and bind specific antigens and serve as essential components of the immune system’s defense against foreign molecules.
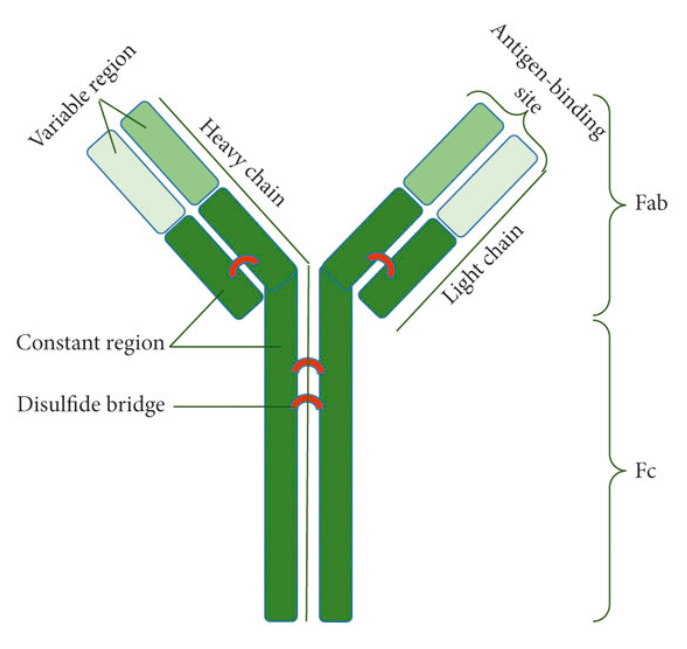
Structurally, antibodies are Y-shaped globular proteins composed of four polypeptide chains—two heavy chains and two light chains—linked by disulfide bonds. Each heavy and light chain contains a variable region and a constant region. The molecule is divided into two major functional domains: the Fab region, which is responsible for antigen recognition, and the Fc region, which engages immune cell receptors or the complement system to mediate downstream effector functions.
Antibody Classes: From Conventional Monoclonal Antibodies to Multifunctional Fusion Modalities
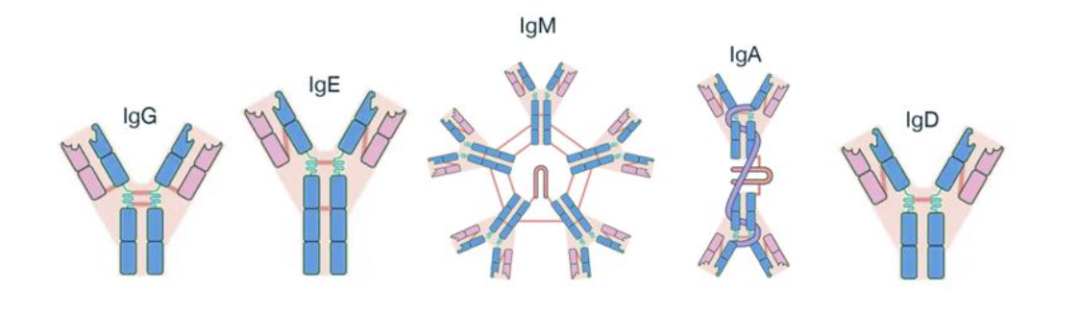
Based on the structural differences in the heavy chain constant region, immunoglobulins are generally classified into five types: IgG, IgM, IgA, IgD, and IgE. Among these, IgG is the most abundant in the human body, possesses a relatively long half-life, and serves as the primary structural basis for current antibody drug design.
Depending on whether the Fc region is retained and the intended engineering purpose or function, IgG and its derivatives are commonly categorized in the literature into several formats: conventional full-length IgG, antibody fragments such as Fab and scFv, bispecific or multispecific antibodies in IgG-like and non-IgG-like formats, antibody–drug conjugates (ADC), as well as Fc-fusion proteins and other immune conjugates. An illustration is shown below:
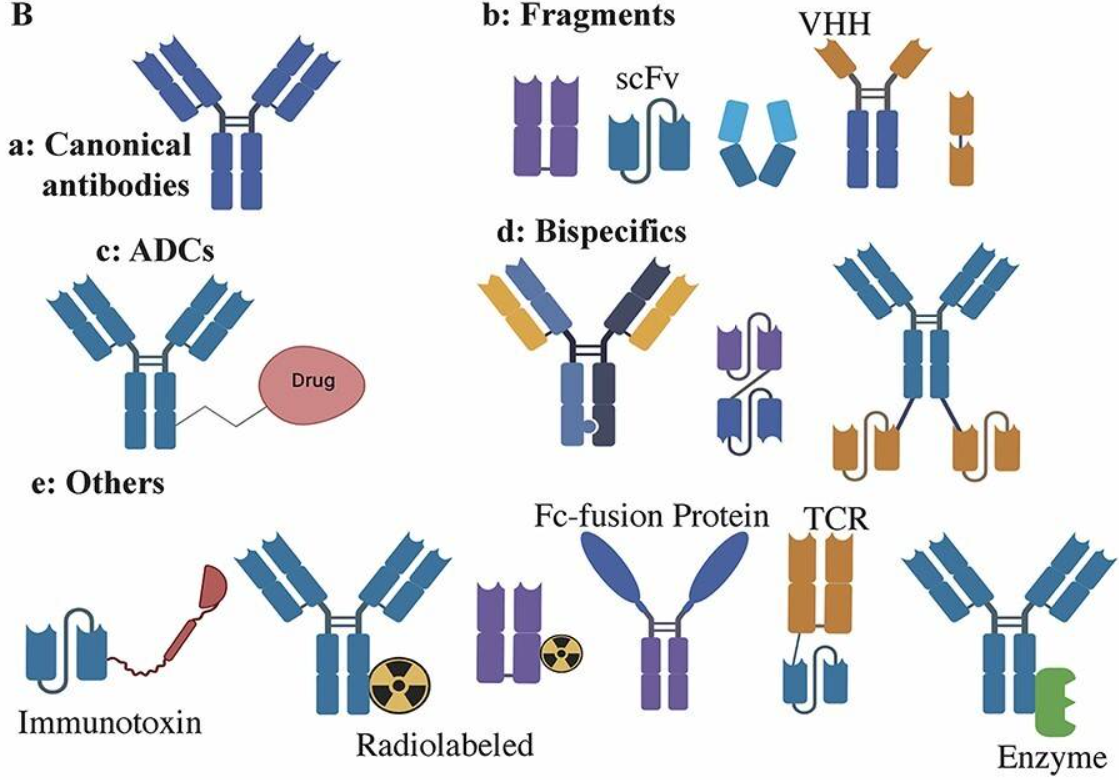
Three Major Antibody Effector Mechanisms: ADCC, ADCP, and CDC
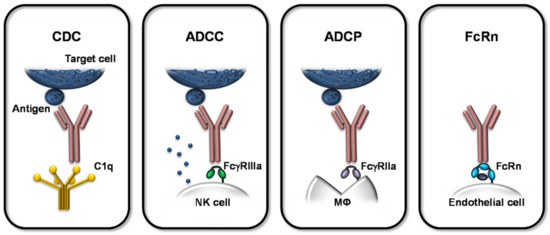
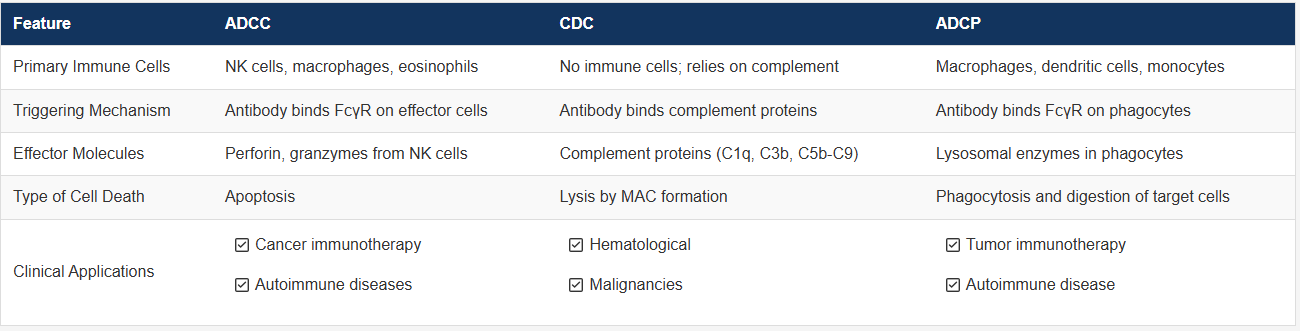
The immune clearance function of antibodies operates on multiple levels. In addition to antigen recognition, effector functions mediated by the Fc region are critical. Among these, antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC) constitute the three principal mechanisms through which antibodies exert their functions.
ADCC
ADCC occurs when the Fab region of an antibody binds to antigenic epitopes on virus-infected or tumor cells, while the Fc region engages Fc receptors on effector cells such as natural killer (NK) cells, macrophages, or neutrophils. This interaction triggers the effector cells to directly kill the target cells and represents a key mechanism for the action of therapeutic antitumor antibodies.
ADCC involves three components: effector cells, antibodies, and target cells. The main Fc receptors on effector cells that interact with IgG include FcγRI (CD64), FcγRII (CD32), and FcγRIIIa (CD16).
ADCP
In contrast to ADCC, ADCP is mediated by phagocytic cells—including monocytes, macrophages, neutrophils, and dendritic cells—that express FcγRIIa (CD32a), FcγRI (CD64), and FcγRIIIa (CD16a). Blocking studies have shown that FcγRIIa is the primary receptor responsible for mediating the phagocytosis of diseased cells.
CDC
The complement system is a group of heat-labile, soluble, and membrane-bound proteins present in mammalian serum and tissue fluids, comprising more than 30 components that exhibit enzymatic activity upon activation. In CDC, antibodies first bind to the complement component C1q, which subsequently activates C2–C9 to form the membrane attack complex (MAC), leading to target cell lysis. Several antitumor antibodies, such as those targeting CD20, CD52, and CEA, can mediate CDC activity.
Fc Engineering of Antibodies
Fc engineering can enhance the tumor-killing potency and immune-activating capacity of antibody therapeutics and has become a key focus in recent antibody drug development. The main strategies include:
C1q-Mediated CDC Enhancement
The Fc region of antibodies can bind to the six globular heads of complement component C1q, initiating a proteolytic cascade of complement proteins in serum, generating inflammatory mediators C3a and C5a, and forming the membrane attack complex (MAC) on the target cell surface, ultimately inducing CDC. Glycoengineering at the N297 residue of the Fc region can strengthen Fc binding to FcγR and C1q, thereby enhancing CDC activity. In studies of autoimmune diseases, introducing mutations such as L234F/L235E/P331S and removing glycosylation can effectively reduce FcγR affinity, suppressing CDC.
FcγR-Mediated ADCC and ADCP Optimization
For FcγR-mediated ADCC and ADCP, the binding affinity between Fc and activating FcγRs directly affects antibody effector functions. Amino acid mutations and glycoengineering can optimize ADCC and ADCP activities. For example, removing core fucose at Fc N297 improves spatial binding with CD16A, significantly enhancing ADCC. Mutations such as S239D/A330L/I332E have been shown to increase ADCC, while the VLPLL modification (L235V/F243L/R292P/Y300L/P396L) can enhance selective Fc-FcγR interactions, further optimizing effector activity.
FcRn-Mediated Half-Life Extension
Fc binding to the neonatal Fc receptor (FcRn) is pH-dependent, allowing IgG recycling and effectively prolonging antibody circulation in vivo. This strategy increases drug exposure and clinical efficacy, providing stable antibody concentrations to support long-term treatment regimens.
FcγRIIB Modulation
FcγRIIB is the only inhibitory FcγR, and its expression can influence antibody efficacy. Fc engineering that enhances binding of agonistic antitumor antibodies to FcγRIIB can optimize antibody activity and modulate immune responses.
Conclusion
In summary, Fc engineering enables the optimization of CDC, FcγR-mediated ADCC/ADCP, and FcRn-mediated half-life extension, providing powerful technological support for antibody drug development. Genomeditech actively follows these cutting-edge strategies, offering cell lines, ready-to-use antibodies, and custom services related to ADCC, ADCP, and FcRn to meet the needs of drug development and clinical applications.
Reference
Allergy Clin Immunol. (2010). 125(202), S41–S52. https://doi.org/10.1016/j.jaci.2009.09.046
Gogesch, P., Dudek, S., van Zandbergen, G., Waibler, Z., & Anzaghe, M. (2021). The role of Fc receptors on the effectiveness of therapeutic monoclonal antibodies. International Journal of Molecular Sciences, 22(16), 8947. https://doi.org/10.3390/ijms22168947
Jefferis, R. (2012). Antibody Fc engineering for enhanced effector functions. mAbs, 4(3), 346–356. https://doi.org/10.4161/mabs.4.3.19860
Lazar, G. A., Dang, W., Karki, S., Vafa, O., Peng, J., Hyun, L., ... & Desjarlais, J. R. (2006). Engineered antibody Fc variants with improved effector function. Proceedings of the National Academy of Sciences, 103(11), 4005–4010. https://doi.org/10.1073/pnas.0511163103
Lyu, X., Zhao, Q., Hui, J., Wang, T., Lin, M., Wang, K., Zhang, J., Shentu, J., Dalby, P. A., Zhang, H., & Liu, B. (2022). The global landscape of approved antibody therapies. Antibody Therapeutics, 5(4), 233–257. https://doi.org/10.1093/abt/tbac021
Nimmerjahn, F., & Ravetch, J. V. (2006). FcγRIIB in immune regulation. Current Opinion in Immunology, 18(3), 245–252. https://doi.org/10.1016/j.coi.2006.03.004
Nimmerjahn, F., & Ravetch, J. V. (2008). Fcγ receptors as regulators of immune responses. Nature Reviews Immunology, 8(1), 34–47. https://doi.org/10.1038/nri2206
Shields, R. L., Lai, J., Keck, R., O’Connell, L. Y., Hong, K., Meng, Y. G., ... & Presta, L. G. (2001). High-resolution mapping of FcγR binding. Journal of Biological Chemistry, 276(9), 6591–6604. https://doi.org/10.1074/jbc.M009483200
Ferrara, C., Grau, S., Jäger, C., Sondermann, P., Brünker, P., & Waldhauer, I. (2011). Glycoengineering of antibodies for enhanced ADCC. Nature Biotechnology, 29(2), 300–305. https://doi.org/10.1038/nbt.1777
Wang, X., Mathieu, M., & Brezski, R. J. (2018). Fc engineering to improve ADCC/ADCP. Trends in Biotechnology, 36(7), 634–649. https://doi.org/10.1016/j.tibtech.2018.02.009
Diebolder, C. A., Beurskens, F. J., de Jong, R. N., Koning, R. I., Strumane, K., Lindorfer, M. A., ... & Schuurman, J. (2014). Complement-activating IgG hexamers. Proceedings of the National Academy of Sciences, 111(26), E2491–E2500. https://doi.org/10.1073/pnas.1402789111
Datta-Mannan, A., Witcher, D. R., Tang, Y., Watkins, J., & Ebel, W. (2010). FcRn and IgG pharmacokinetics. mAbs, 2(4), 383–390. https://doi.org/10.4161/mabs.2.4.12989
Chu, S. Y., Ercan, A., Li, H., & Li, J. (2020). FcγRIIB engineering for agonistic antibodies. mAbs, 12(1), 1787475. https://doi.org/10.1080/19420862.2020.1787475
Science Notes. (n.d.). Types of antibodies and their functions. https://sciencenotes.org/types-of-antibodies-and-their-functions/
The Perspective of Therapeutic Antibody Marketing in Iran: Trend and Estimation by 2025. (2021). Advances in Pharmacological and Pharmaceutical Sciences, 2021(12), 1–7. https://doi.org/10.1155/2021/5569590