CD276 Overview
CD276 is a type I transmembrane glycoprotein and a member of the B7 family. It was first identified in 2001.
The CD276 gene is located on chromosome 15 and encodes a protein consisting of 316 amino acids, with a relative molecular weight ranging from 45 to 66 kDa. The full-length CD276 protein comprises four regions: a predicted signal peptide, extracellular V- and C-like immunoglobulin domains (IgV and IgC), a transmembrane domain, and a cytoplasmic tail. There are two isoforms: 2Ig CD276 and 4Ig CD276, with the latter being the predominant form.
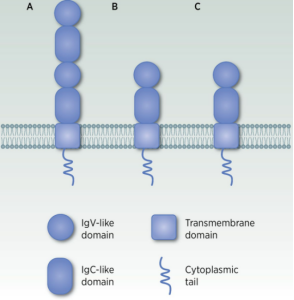
Structure of CD276
Some studies have shown that CD276 exhibits both co-stimulatory and inhibitory functions in the immune system within non-malignant tissues. On one hand, CD276 acts as a co-stimulatory molecule, promoting T cell activation, proliferation, and the secretion of cytokines such as IFN-γ and IL-2. On the other hand, CD276 also plays a co-inhibitory role in certain adaptive immune responses, enabling tumor cells to evade immune surveillance and suppressing NK cell–mediated cytolysis.
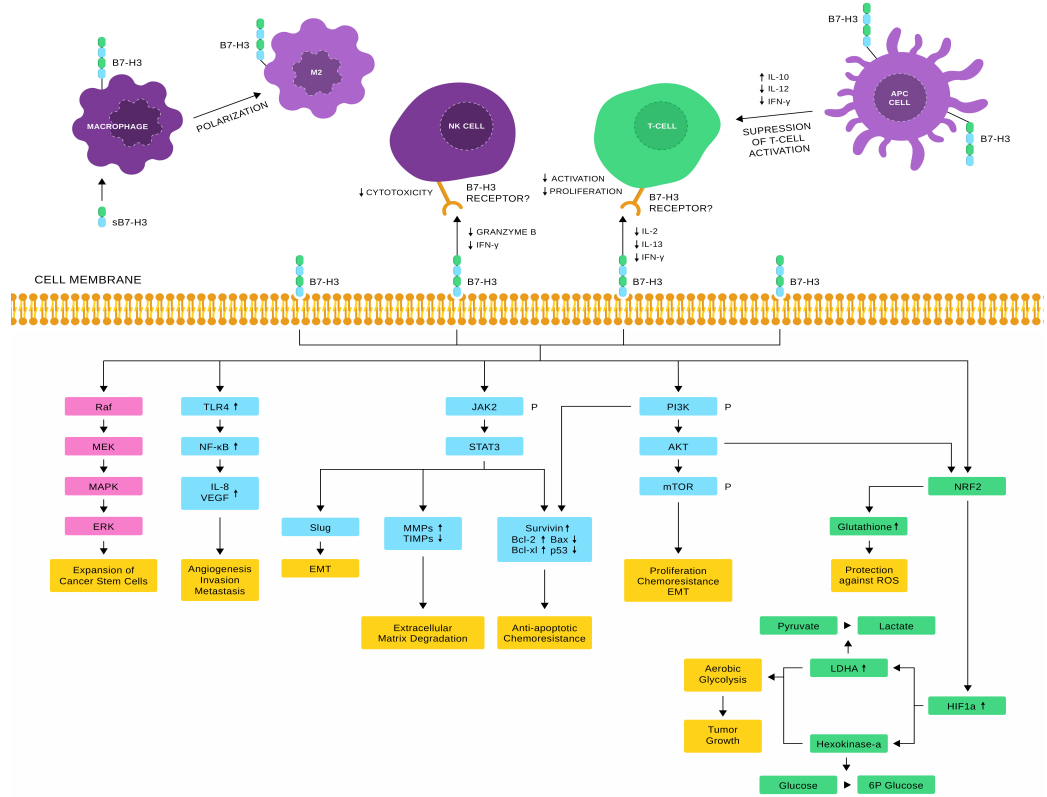
Signaling Pathways of CD276
Studies have shown that CD276 is overexpressed in various tumor types, including non-small cell lung cancer, pancreatic cancer, and primary liver cancer. Its elevated expression in tumor tissues is often associated with poor prognosis, shorter overall survival (OS), and reduced progression-free survival (PFS). In lung cancer—particularly small cell lung cancer—approximately 65% of patients exhibit high CD276 expression in their tumors. This overexpression correlates with poor clinical outcomes, highlighting CD276 as a promising therapeutic target.

Expression of CD276 in Cancer Tissues
Current Research Landscape of CD276
To date, no CD276 ADC products have been approved for market release worldwide. The research and development landscape for CD276 ADCs differs from that of other therapeutic areas. Despite challenges encountered during development, global companies continue to make persistent efforts to advance this field.
According to research, there are currently seven CD276 ADC candidates in clinical development worldwide, targeting a broad range of cancers, including neuroblastoma with CNS involvement, lung cancers, sarcoma, head and neck cancers, as well as other advanced or recurrent solid tumors.
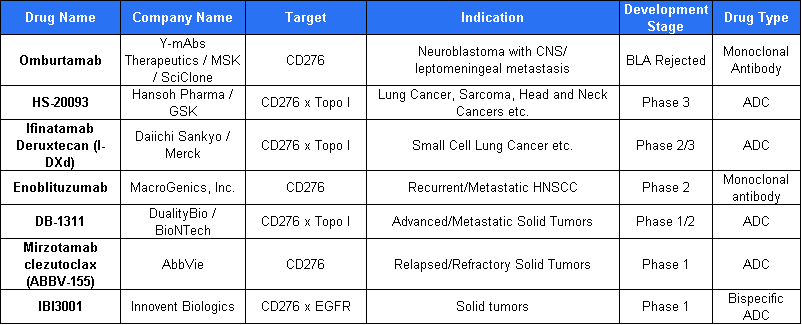
Inatuzumab Deruxtecan
Co-developed by Daiichi Sankyo and Merck & Co., this antibody-drug conjugate has entered a global Phase III clinical trial for prostate cancer, becoming the first CD276-targeting ADC to reach this stage of development.
Built on Daiichi Sankyo’s proprietary DXd platform, the candidate represents a significant advancement in the field and highlights the growing momentum in the global CD276 ADC landscape.
HS-20093
HS-20093 is a CD276-targeting antibody-drug conjugate (ADC) that utilizes a clinically validated topoisomerase inhibitor (TOPOi) as its cytotoxic payload.
On December 20, 2023, GSK entered into a licensing agreement with Hansoh Pharma, securing the ex-China rights to HS-20093. The deal includes an upfront payment of $185 million, with potential milestone payments of up to $1.525 billion. Following the agreement, GSK has initiated two Phase III clinical trials, focusing on small cell lung cancer and osteosarcoma.
As of April 18, 2025, the Chinese Drug Clinical Trial Registry (CTR20251474) indicates that Hansoh has registered a Phase III clinical trial comparing HS-20093 injection with gemcitabine plus docetaxel in patients with osteosarcoma who have failed second-line treatment. This marks the first Phase III osteosarcoma trial initiated for this candidate.
Recently, HS-20093 has entered a Phase I clinical trial in combination with a PD-L1 antibody as a first-line maintenance therapy for small cell lung cancer. This combination strategy is expected to enhance response rates through immune-synergistic mechanisms, offering a potential new treatment option for tumors with low immunogenicity.
Conclusion
CD276 has rapidly gained attention as a compelling target in oncology, driven by its high expression in a range of solid tumors and its role in immune modulation. Although no CD276-targeting ADCs have been approved to date, several candidates, including Inatuzumab deruxtecan
and HS-20093, have advanced into late-stage clinical development, signaling strong momentum in the field. With ongoing innovations in payload design and combination strategies, CD276 ADCs hold significant potential to become an important therapeutic option for difficult-to-treat cancers.
Reference
BioNTech. (2024). BioNTech and DualityBio receive FDA Fast Track designation for Antibody-Drug Conjugate candidate BNT324/DB-1311 in prostate cancer. https://investors.biontech.de/news-releases/news-release-details/biontech-and-dualitybio-receive-fda-fast-track-designation/
Carneiro, B. A., Perets, R., Dowlati, A., LoRusso, P., Yonemori, K., He, L., Munasinghe, W., Noorani, B., Johnson, E. F., & Zugazagoitia, J. (2023). Mirzotamab clezutoclax as monotherapy and in combination with taxane therapy in relapsed/refractory solid tumors: Dose expansion results. Journal of Clinical Oncology, 41(16), 3027. https://doi.org/10.1200/jco.2023.41.16_suppl.3027
Chapoval, A., Ni, J., Lau, J. et al. B7-H3: A costimulatory molecule for T cell activation and IFN-γ production. Nat Immunol 2, 269–274 (2001). https://doi.org/10.1038/85339
GSK. (2023). GSK enters exclusive license agreement with Hansoh for HS-20093. https://www.gsk.com/en-gb/media/press-releases/gsk-enters-exclusive-license-agreement-with-hansoh-for-hs-20093/
Innoventbio. (2024). Innovent to present preclinical data of multiple novel molecules at the 2024 AACR Annual Meeting. https://www.innoventbio.com/InvestorsAndMedia/PressReleaseDetail?key=442
Kontos, F., Michelakos, T., Kurokawa, T., Sadagopan, A., Schwab, J. H., Ferrone, C. R., & Ferrone, S. (2021). B7-H3: An Attractive Target for Antibody-based Immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research, 27(5), 1227–1235. https://doi.org/10.1158/1078-0432.CCR-20-2584
Koumprentziotis, I. A., Theocharopoulos, C., Foteinou, D., Angeli, E., Anastasopoulou, A., Gogas, H., & Ziogas, D. C. (2024). New Emerging Targets in Cancer Immunotherapy: The Role of B7-H3. Vaccines, 12(1), 54. https://doi.org/10.3390/vaccines12010054
MacroGenics. (2022). MacroGenics Announces Closure of CP-MGA271-06 Study Evaluating Enoblituzumab plus Checkpoint Inhibition in Head and Neck Cancer. https://ir.macrogenics.com/news-releases/news-release-details/macrogenics-announces-closure-cp-mga271-06-study-evaluating
Merck. (2024). Ifinatamab Deruxtecan Continues to Demonstrate Promising Objective Response Rates in Patients with Extensive-Stage Small Cell Lung Cancer in IDeate-Lung01 Phase 2 Trial. https://www.merck.com/news/ifinatamab-deruxtecan-continues-to-demonstrate-promising-objective-response-rates-in-patients-with-extensive-stage-small-cell-lung-cancer-in-ideate-lung01-phase-2-trial/
Y-mAbs Therapeutics, Inc. (2020). Y-MABS announces completion of submission of Omburtamab Biologics License Application to FDA. https://ir.ymabs.com/news-releases/news-release-details/y-mabs-announces-completion-submission-omburtamab-biologics/