Sanofi’s amlitelimab met all primary and key secondary endpoints in the COAST 1 phase 3 study in adults and adolescents with atopic dermatitis
Amlitelimab, dosed every four weeks or every 12 weeks, demonstrated statistically significant and clinically meaningful efficacy in skin clearance and disease severity compared to placebo at Week 24, with efficacy progressively increasing throughout the treatment period
Study results reinforce the potential of amlitelimab as the first and only atopic dermatitis treatment with possible dosing of only four times per year
Additional phase 3 data will provide a comprehensive understanding of amlitelimab’s efficacy and safety profile, including the role of long-term maintenance treatment and the potential for off-treatment efficacy across diverse treatment populations
September 4, 2025. Positive results from the global COAST 1 phase 3 study (clinical study identifier: NCT06130566) showed that amlitelimab, a fully human non-T cell depleting monoclonal antibody that targets OX40-ligand (OX40L), dosed either every four weeks (Q4W) or every 12 weeks (Q12W), met all primary and key secondary endpoints, demonstrating statistically significant and clinically meaningful skin clearance and disease severity compared to placebo at Week 24 in patients aged 12 years and older with moderate-to-severe atopic dermatitis (AD). Amlitelimab was well-tolerated, with no new safety concerns identified in this study.Learn more about our catalog.
The key endpoints were measured at Week 24 in patients who received amlitelimab either Q4W or Q12W. For US and US reference countries, the primary endpoint was the proportion of patients with a validated investigator global assessment scale for AD (vIGA-AD) of 0 (clear) or 1 (almost clear) and a reduction from baseline score of ≥2 points. For the EU, EU reference countries and Japan, the co-primary endpoints comprised the proportion of patients with vIGA-AD 0/1 and a reduction from baseline score of ≥2 points along with the proportion of patients reaching a 75% or greater improvement in the eczema area and severity index total score (EASI-75).
| Key endpoints Proportion of patients | Non-responder imputation* | Treatment policy** | ||||
| Q4W | Q12W | Placebo | Q4W | Q12W | Placebo | |
| vIGA-AD 0/1 | 21.1% p-value (p) <0.01 | 22.5% p <0.01 | 9.2% | 26.5% p <0.001 | 29.1% p <0.001 | 10.5% |
| EASI-75 | 35.9% p <0.001 | 39.1% p <0.001 | 19.1% | 46.0% p <0.001 | 50.3% p <0.001 | 27.6% |
* Non-responder imputation: includes patients with rescue/prohibited medication use prior to Week 24 and missing data.
** Treatment policy: includes data for patients with rescue medication use prior to Week 24. Note: In both analyses non-responder imputation for patients with prohibited medication use and missing data.
In both treatment arms, a progressive increase in efficacy without plateau was observed during the treatment period:
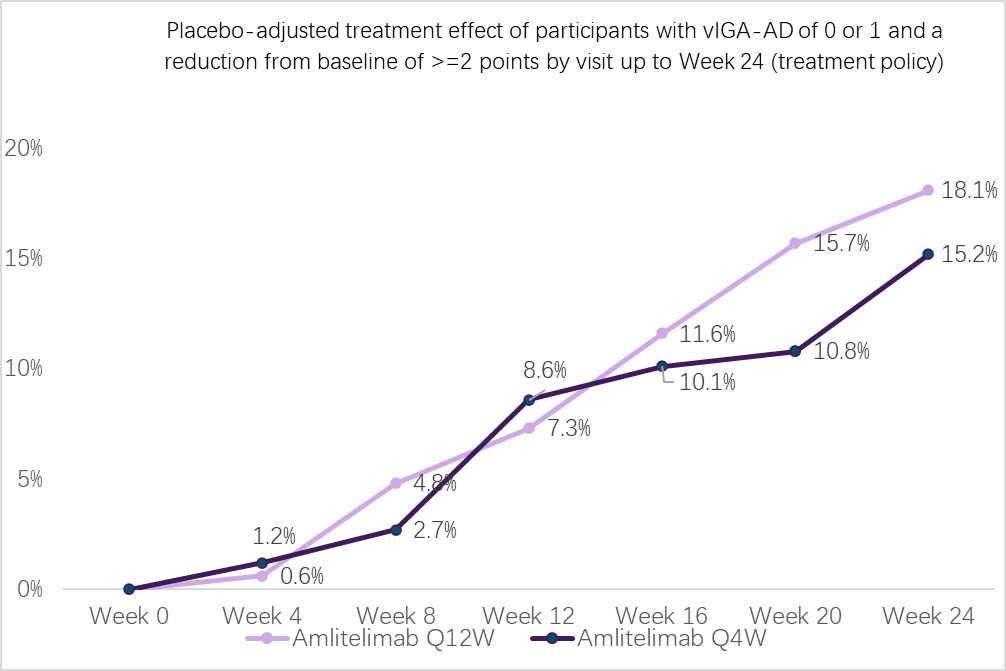
(Treatment effects at Week 24 are modeled and do not reconcile with the table).
The study’s key secondary endpoints were also achieved across both dosing arms at Week 24, including the proportion of patients who achieved a vIGA-AD 0/1 with only barely perceptible erythema and a reduction from baseline of ≥2-points, and the proportion of patients who achieved a ≥4-point reduction in peak pruritus-numerical rating scale (PP-NRS) from baseline in patients with a baseline PP-NRS ≥4.
The most common treatment emergent adverse events (TEAEs) in COAST 1 (≥5% in any dose arm) were AD, nasopharyngitis and upper respiratory tract infection. All were more common in the placebo arm compared to amlitelimab-treated arms. Injection site reactions were numerically higher in amlitelimab arms (pooled amlitelimab 2.2%, placebo 0.7%). All were mild, patients recovered, and study medication was continued in all cases. Rates of pyrexia (1.1% in pooled amlitelimab arms vs. 0.7% in placebo arm) and chills (0.4% in pooled amlitelimab arms vs. 0% in placebo arm) were low. Overall, rates of treatment-emergent adverse events (TEAEs), serious adverse events, and TEAEs resulting in treatment discontinuation were similar in the placebo arm and pooled amlitelimab arms.
Full results will be submitted for presentation at a forthcoming medical meeting.
The OCEANA clinical development program of amlitelimab in AD, which includes COAST 1 and four other phase 3 studies (SHORE, COAST 2, AQUA, and ESTUARY) is anticipated to read out through 2026 and comprises the foundation for potential global regulatory submissions.
Amlitelimab is currently under clinical investigation, and its safety and efficacy have not been evaluated by any regulatory authority.
About the COAST 1 study
COAST 1 was a randomized, double-blind, placebo-controlled, parallel-group, 3-arm, global, multicenter phase 3 study to evaluate the efficacy and safety of amlitelimab monotherapy by subcutaneous injection in 601 adults and adolescents aged 12 years and older with moderate-to-severe AD. Key objectives included measuring the efficacy and safety of amlitelimab compared to placebo at Week 24. In the study, amlitelimab was administered at a dose of 250 mg (125 mg for those with body weight <40 kg) on either a Q4W or Q12W schedule following a loading dose of 500 mg (250 mg for those with body weight <40 kg). The study included sites in 15 countries across North America, Latin America, Europe, Asia-Pacific and the Middle East, reflecting a diverse study population.
About amlitelimab
Amlitelimab (SAR445229, KY1005) is a fully human, non-T cell depleting monoclonal antibody that blocks OX40L, a key immune regulator. With its novel mechanism of action, amlitelimab aims to normalize the overactive immune system, without depleting T cells. It has the potential to be a first- or best-in-class treatment for a range of immune-mediated diseases and inflammatory disorders, including the anchor indication of moderate-to-severe AD, and potentially in moderate-to-severe asthma, systemic sclerosis, celiac disease, and alopecia areata.














