On May 14, 2025, GSK announced plans to acquire the core assets of Boston Pharmaceuticals, including efimosfermin alfa, for up to $2 billion in cash. Efimosfermin alfa is a long-acting FGF21 analog administered once monthly via subcutaneous injection, with multiple potential benefits including metabolic regulation, liver fat reduction, inflammation improvement, and fibrosis reversal.
On September 18, Roche further intensified the competition by announcing the acquisition of 89bio and its flagship product, pegozafermin, another FGF21 analog, targeting patients with moderate-to-severe fibrosis (F2–F3) and cirrhosis (F4) in MASH.
Within just a few months, two major multinational corporations engaged in high-value cash deals to secure the same target, underscoring the potential and hype surrounding FGF21. It has emerged as one of the hottest targets in metabolic disease research, following the success of GLP-1.
So, what exactly is FGF21, and why does it hold such high expectations?
FGF21: Unique Mechanisms for Multi-Organ Metabolic Regulation
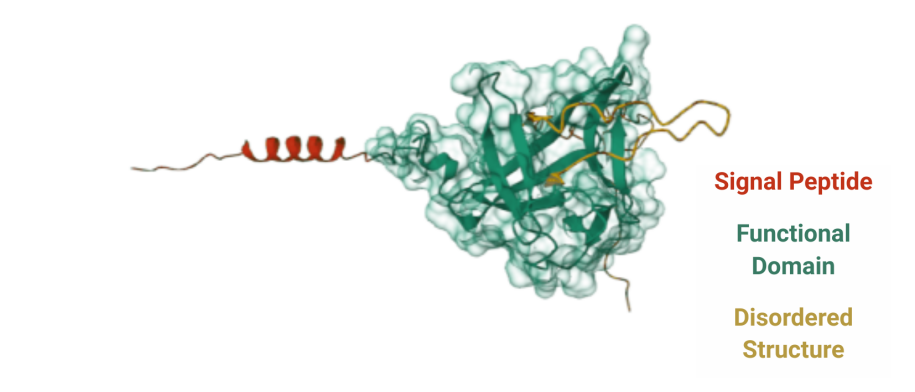
FGF21 (Fibroblast Growth Factor 21) is an endocrine member of the FGF superfamily, alongside FGF19 and FGF23, forming a group of key factors with important roles in metabolic regulation. FGF21 is composed of 209 amino acids and is classified as an endocrine extracellular protein. Its signal peptide spans residues 1–28, directing secretion, while the main functional domain covers residues 29–216. The C-terminal region contains an intrinsically disordered segment that is susceptible to inactivation via FAP-mediated cleavage.
FGF21 is primarily synthesized and secreted by the liver, though extrahepatic tissues such as white adipose tissue, brown adipose tissue, and skeletal muscle also exhibit a certain level of expression. As a metabolic regulator, FGF21 plays a central role in maintaining glucose homeostasis, enhancing insulin sensitivity, and promoting fatty acid oxidation.
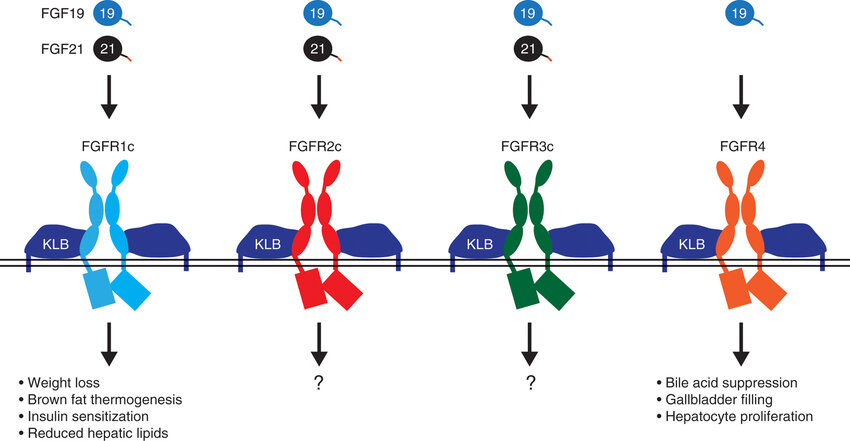
FGF21 requires the specific co-receptor β-Klotho (KLB) to activate the “c” splice isoforms of FGFR1, FGFR2, and FGFR3. β-Klotho is a single-pass transmembrane protein that specifically recognizes FGF21 and forms a receptor complex with FGFRs to mediate signal transduction. In the absence of β-Klotho, FGF21 cannot directly bind to FGFRs, making β-Klotho a key factor in mediating the biological functions of FGF21.
FGF21 exerts precise effects on the core pathological features of MASH through its multiple and synergistic mechanisms of action. In the liver, FGF21 directly reduces hepatic fat content (hepatic steatosis) and significantly improves insulin sensitivity, thereby alleviating hepatocellular injury at its source. In adipose tissue, it effectively promotes lipolysis and energy expenditure, contributing to overall improvements in systemic metabolic health. Notably, FGF21 also acts on the central nervous system, regulating appetite and energy preferences, and significantly reducing cravings for sugar and alcohol—a mechanism that shows a marked synergistic effect with GLP-1–based therapies.
Unlike GLP-1, which primarily targets weight loss and glycemic control, FGF21 analogs demonstrate the potential to address the core pathologies of MASH in a “one-stop” manner: markedly reducing hepatic fat, improving inflammation, and directly reversing liver fibrosis—the latter being a key determinant of prognosis in MASH patients.
Nonalcoholic Fatty Liver Disease: The Metabolic Battlefield for Pharma
Steatotic liver disease (SLD) is a pathological condition characterized by the accumulation of fat in the liver and has become a major global health challenge. Data indicate that the most prevalent subtype, metabolic dysfunction-associated steatotic liver disease (MASLD), affects approximately 25% of the adult population worldwide.
As MASLD progresses to the more severe metabolic dysfunction-associated steatohepatitis (MASH), the liver undergoes pronounced inflammation and fibrosis, which can further lead to cirrhosis, liver failure, and even hepatocellular carcinoma. The disease burden is substantial: in the United States, MASH and alcohol-associated liver disease (ALD) are now leading causes of liver transplantation, with significant healthcare costs. In China, with lifestyle changes and rising obesity rates, the prevalence of steatotic liver disease has been increasing steadily, making it one of the most common liver disorders.
The combination of a large patient population and urgent unmet treatment needs constitutes a key driver for pharmaceutical giants to invest heavily in this field.
Global Landscape of FGF21 R&D: Who Is Leading the Race?
Globally, companies developing therapies targeting FGF21 include GSK, Akero Therapeutics, and 89bio.
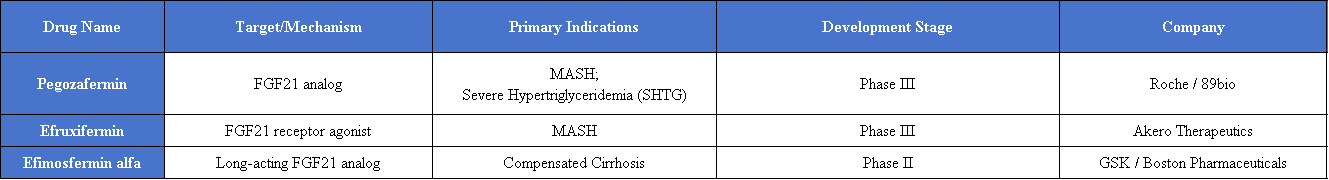
89bio (now part of Roche): Pegozafermin
Pegozafermin is an FGF21 analog currently in phase III clinical trials, focusing on the treatment of moderate-to-severe metabolic dysfunction-associated steatohepatitis (MASH). Its development particularly targets patients with F2 and F3 liver fibrosis as well as those with F4 cirrhosis.
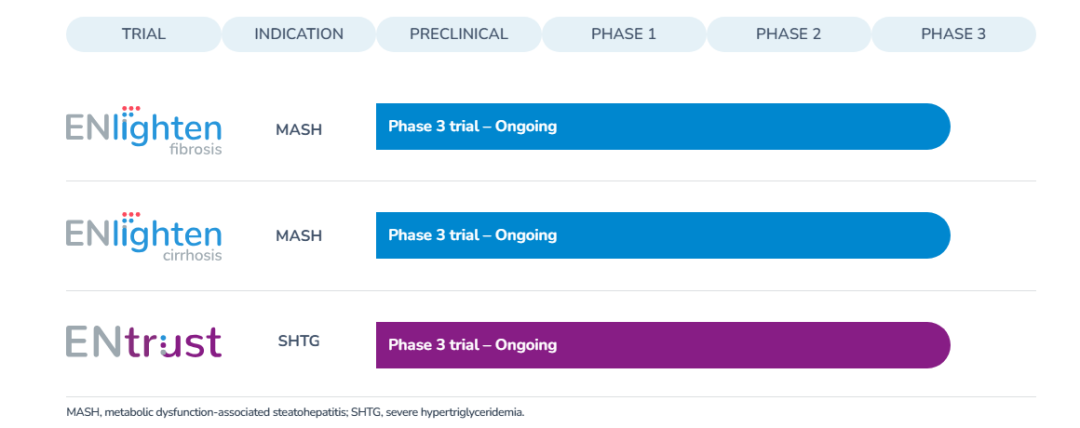
The molecule incorporates a proprietary glyco-PEGylation technology, which enhances its bioactivity and extends its half-life, thereby improving therapeutic durability and patient convenience. Through this unique mechanism, Pegozafermin holds strong potential to become a leading therapeutic candidate for MASH, particularly in patients with advanced fibrosis and cirrhosis, where effective treatment options remain limited.
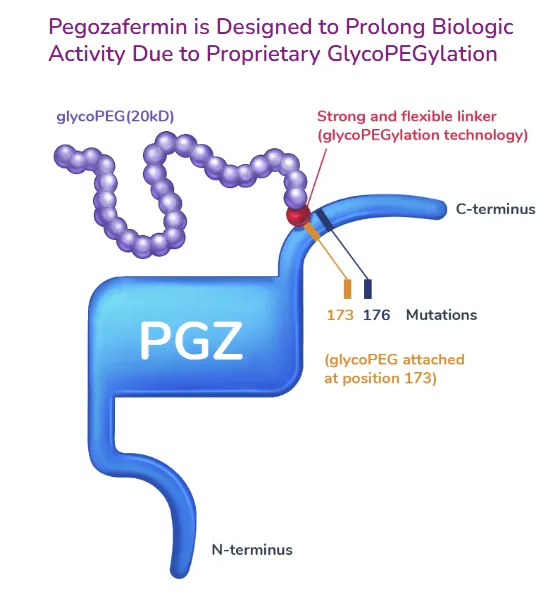
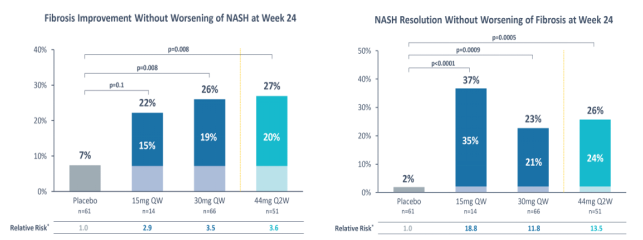
In the phase 1b/2a proof-of-concept trial of Pegozafermin for MASH, treatment with weekly or biweekly dosing achieved nearly a 30% reduction in liver fat fraction in about 88% of patients at week 13 compared with placebo. In addition, improvements were observed in liver transaminases, fibrosis, and lipid biomarkers. The therapy was well tolerated, with no treatment-related serious adverse events reported.
GSK: Efimosfermin alfa
In May 2025, GSK acquired Boston Pharmaceuticals’ long-acting FGF21 analog Efimosfermin alfa. This molecule demonstrates unique advantages in improving liver fibrosis and reversing early cirrhosis.
Efimosfermin exerts its effects by activating the FGFR1/β-Klotho receptor complex in hepatocytes, which in turn activates the phosphatase PPP6C and inhibits the mTORC1 signaling pathway. This mechanism effectively reduces lipid synthesis while promoting fatty acid oxidation. In parallel, Efimosfermin modulates sympathetic nervous system activity via the central nervous system, indirectly suppressing hepatic lipogenesis.
In a phase II clinical trial, Efimosfermin treatment significantly increased the resolution rate of MASH (67.7% vs. 29.4% in the placebo group). Importantly, this efficacy was independent of GLP-1–based background therapies, highlighting its strong potential as a foundational treatment option.
Conclusion and Outlook
With accelerating R&D progress, FGF21 has rapidly emerged as a key focus for major pharmaceutical companies. Its multiple advantages—including regulation of glucose and lipid metabolism, enhancement of insulin sensitivity, and improvement of liver fibrosis—directly address substantial unmet clinical needs. Continuous investment from global players is driving this pathway from the research frontier into clinical practice and the market.
Looking ahead, FGF21 is poised to become a central therapeutic avenue not only for MASH but also for a broader range of metabolic diseases, reshaping the future landscape of metabolic drug development. Backed by multi-billion-dollar commitments from industry leaders, FGF21 is no longer just a promise—the momentum has truly arrived.
Reference
Akerotx. (2025). Efruxifermin. https://akerotx.com/efruxifermin/
GSK. (2025, May 14). GSK to acquire efimosfermin, a phase III-ready potential best-in-class specialty medicine to treat and prevent progression of steatotic liver disease (SLD). https://www.gsk.com/en-gb/media/press-releases/gsk-to-acquire-efimosfermin-a-phase-iii-ready-potential-best-in-class-specialty-medicine-to-treat-and-prevent-progression-of-steatotic-liver-disease-sld/
Harrison, S. A., et al. (2023). Pegozafermin in patients with non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2b trial. The Lancet.
Itoh, N. (2010). Hormone-like (endocrine) Fgfs: their evolutionary history and roles in development, metabolism, and disease. Cell and Tissue Research, 342(1), 1–11.
Kharitonenkov, A., et al. (2005). FGF-21 as a novel metabolic regulator. Journal of Clinical Investigation, 115(6), 1627–1635.
Kurosu, H., et al. (2007). Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. Journal of Biological Chemistry, 282(37), 26687–26695.
Ogawa, Y., et al. (2007). BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proceedings of the National Academy of Sciences, 104(6), 2432–2437.
Potthoff, M. J., et al. (2012). FGF21 and the pathophysiology of metabolic syndrome. Applied Physiology, Nutrition, and Metabolism, 37(4), 672–679.
Powell, E. E., Wong, V. W., & Rinella, M. (2021). Non-alcoholic fatty liver disease. The Lancet, 397(10290), 2212–2224.
Roche. (2025, September 18). Roche to acquire liver drug developer 89bio for up to $3.5 billion. https://www.roche.com/media/releases/med-cor-2025-09-18
Talukdar, S., et al. (2016). FGF21: A novel regulator of glucose and lipid metabolism and whole-body energy balance. Nature Reviews Endocrinology, 12(1), 57–64.
Younossi, Z. M., et al. (2016). Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology, 64(1), 73–84.
Zhou, F., Zhou, J., & Wang, W. (2019). The changing landscape of liver disease in China: a review of the global burden of disease and evidence-based recommendations for its management. Hepatology International, 13(5), 1–11.














