The 2025 World Conference on Lung Cancer (WCLC), hosted by the International Association for the Study of Lung Cancer (IASLC), convened from September 6-9 in Barcelona, Spain. At this conference, two studies on iza-bren (BL-B01D1)-a first-in-class EGFR×HER3 bispecific antibody-drug conjugate (ADC) independently developed by Biokin Pharma-were presented as oral reports, demonstrating its efficacy in non-small cell lung cancer (NSCLC). The data further validate iza-bren’s promising clinical potential and reliability for EGFR-mutant NSCLC populations.
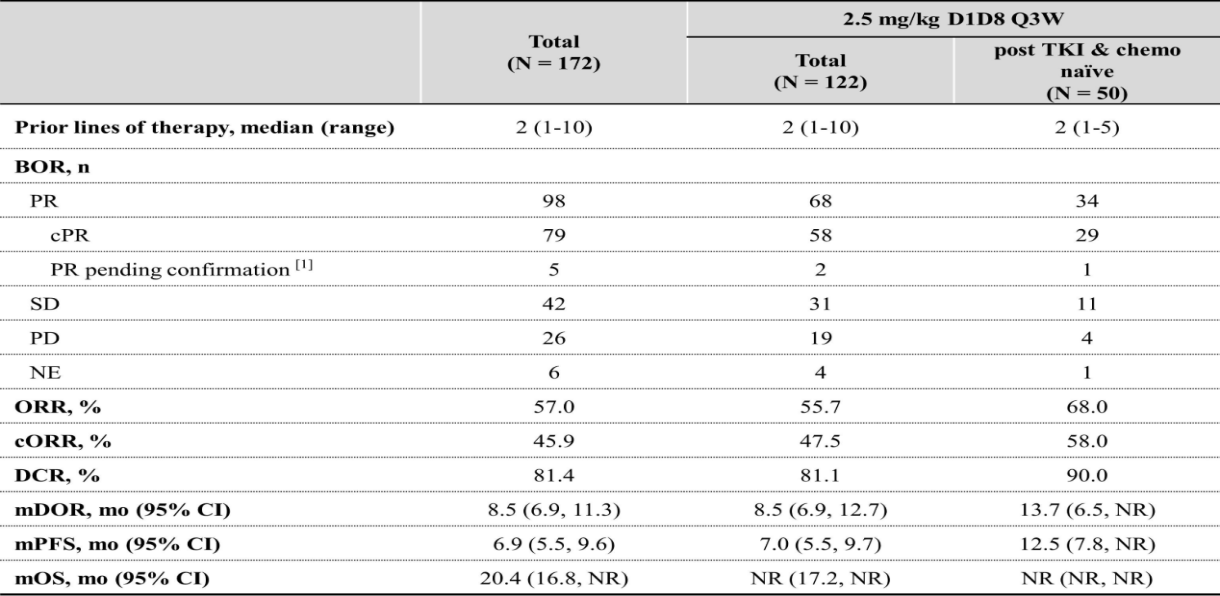
This time, WCLC announced the I/II studies on iza-bren monotherapy for previously treated advanced or metastatic EGFR-mutated NSCLC. A total of 172 EGFR-mutant NSCLC patients (pts) were enrolled, The median follow-up was 15.4 months. Among them, 50 pts who were post TKI and chemo-therapy naïve were treated at 2.5 mg/kg D1D8 Q3W. In this subgroup, ORR was 68.0%, cORR was 58.0%, mPFS was 12.5 months, mDOR was 13.7 months, and mOS was not reached.
Third-generation EGFR-TKIs are the standard first-line therapy for EGFR-mutant NSCLC, yet nearly all patients develop resistance. Subsequent options are limited, with mPFS typically just 4-6 months. But the mPFS of this study reached 12.5 months, which is the longest mPFS reported for ADC drugs to date.
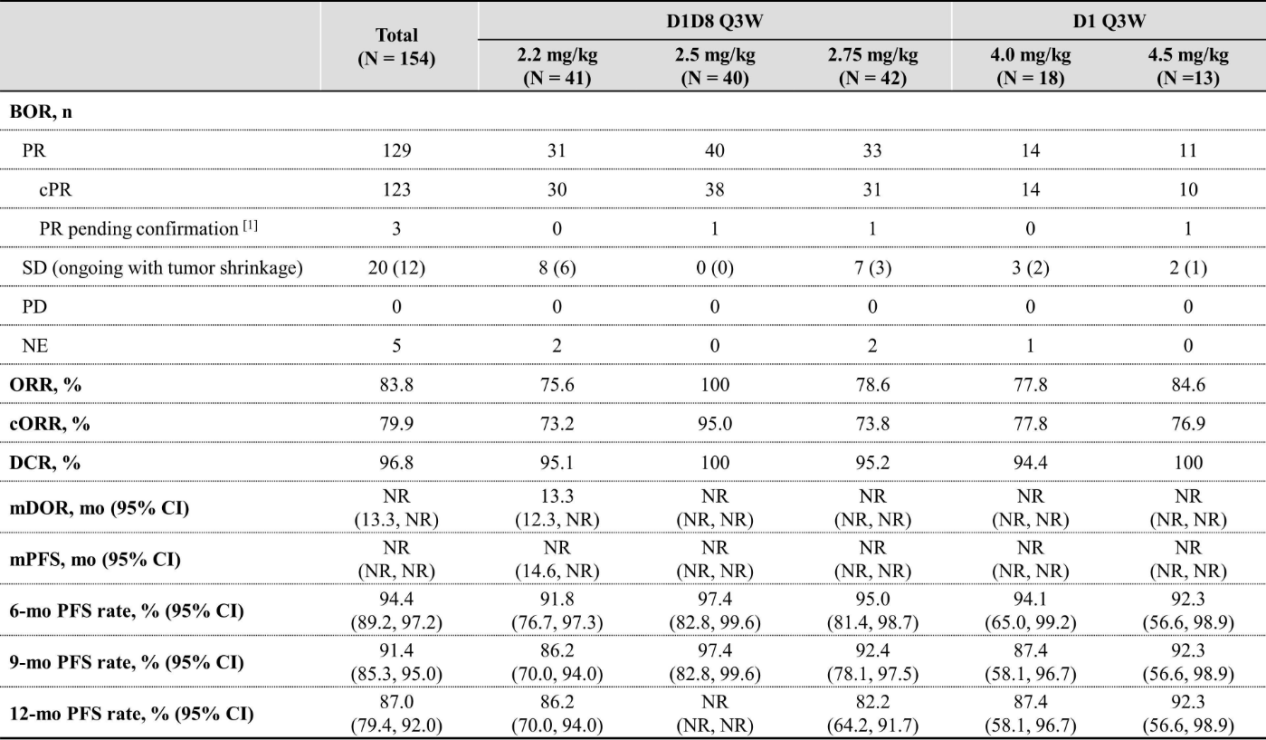
Another phase II study explored the efficacy and safety of different dosing regimens of iza-bren combined with osimertinib (Osi) as first-line treatment for EGFR-mutated NSCLC pts. Among 40 pts at 2.5 mg/kg plus Osi, ORR was 100%, cORR was 95.0% with 1 PR pending confirmation, median DOR and PFS were not reached, and 9-months and 12-months PFS rate were 97.4% and not reached. The results of this study are remarkable, suggesting that this combination regimen has the potential to become a first-line treatment option for EGFR-mutated NSCLC.
Unveiling the Molecular Mechanism of EGFR and HER3
The ERBB receptor family comprises four members (EGFR/HER1, HER2, HER3, and HER4), whose aberrant activation in various cancers has made them critical targets for precision therapy. EGFR possesses full tyrosine kinase activity. Upon binding to its ligands, it dimerizes, activating downstream signaling pathways such as RAS-RAF-MEK-ERK and PI3K-AKT-mTOR, which promote tumor cell proliferation and metastasis.
HER3, once considered to have limited therapeutic value due to defects in its kinase domain, is now recognized as an indispensable dimerization partner within the EGFR family. It contains six binding sites of PI3K, making it the most potent ERBB member for activating the PI3K-AKT pathway. During EGFR-TKI treatment, tumor cells develop resistance through several mechanisms: forming heterodimers with other receptors like MET or AXL; upregulating HER3 expression; and activating ligand autocrine loops, such as heregulin.
Notably, EGFR and HER3 can form a functionally complementary heterodimer: EGFR provides the kinase activity, while HER3 provides the docking sites for signaling adaptor proteins. This synergy leads to potent activation of both the PI3K/AKT and MAPK pathways, driving tumor progression. This mechanism provides a rationale for simultaneously targeting both receptors, suggesting that synergistic inhibition could effectively overcome resistance associated with monotherapies.
The development and bottlenecks of EGFR targeted therapy
EGFR-targeted therapies primarily include small-molecule inhibitors (TKIs) directed against the intracellular kinase domain and monoclonal antibodies targeting the extracellular region. TKIs competitively inhibit ATP binding, thereby blocking EGFR autophosphorylation and downstream signal transduction.
Currently, EGFR-TKIs have advanced to the fourth generation, with the third-generation drug osimertinib established as the first-line standard therapy, demonstrating a median progression-free survival (mPFS) of 18.9 months.
Nevertheless, acquired resistance remains a major challenge, with the C797S mutation accounting for approximately 15-20% of resistance mechanisms.
Despite iterative updates to EGFR-TKIs, resistance continues to be a significant issue. Platinum-based doublet chemotherapy, the standard subsequent regimen, achieves an mPFS of only 4-5 months and is associated with significant toxicity.
Breaking through traditional combination: Bispecific antibodies usher in a new paradigm of treatment
In recent years, innovative combined strategies such as the four-drug combination of immunotherapy+chemotherapy+anti-angiogenesis, the three-drug combination of bispecific antibody+chemotherapy, and ADC drugs have increased the mPFS to 6.3-7.2 months, but still fail to meet clinical needs. Bispecific antibodies provide a new idea for overcoming drug resistance by simultaneously targeting EGFR and HER3.
Currently, there are 14 targeted EGFR×HER3 bispecific ADCs in development, and 6 are in clinical stages, involving well-known ADC enterprises such as Biokin Pharma, Duality Biotherapeutics, Junshi Biosciences, and Innovent Biologics.
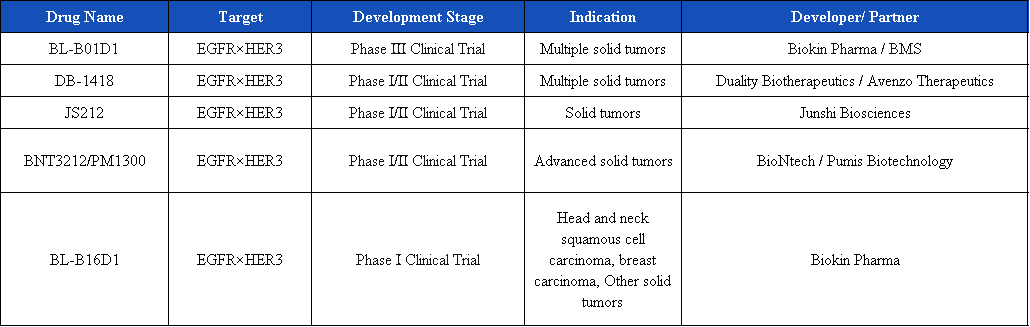
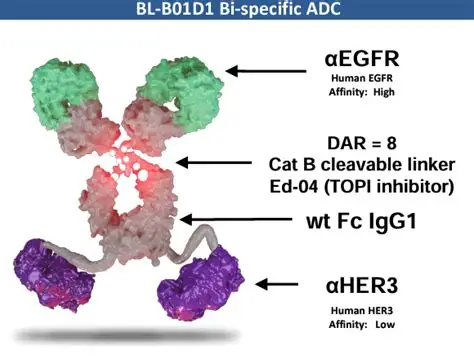
Iza-bren (BL-B01D1)
Iza-bren (BL-B01D1) is the world’s first and only EGFR×HER3 bispecific antibody-drug conjugate (ADC) to advance to Phase III clinical development. It employs a dual mechanism of action:
Signal blockade
Simultaneously inhibits EGFR and HER3 signaling transduction to tumor cells, suppressing proliferation and survival pathways.
Payload delivery
Following antibody-mediated internalization, it releases a cytotoxic payload that induces genotoxic stress, ultimately triggering tumor cell death.
At the 2025 WCLC conference, Biokin Pharma announced that the objective response rate (ORR) of Iza-bren in combination with osimertinib for first-line treatment was as high as 100%, and the median progression-free survival (mPFS) of the second-line monotherapy reached over 12 months! This landmark breakthrough is expected to drive a comprehensive revolution in the diagnosis and treatment of advanced non-small cell lung cancer (NSCLC) with EGFR mutations. Currently, the phase III studies of Iza-bren monotherapy for treatment after the resistance to third-generation TKIs and its first-line combination therapy with osimertinib have been initiated in China.
DB-1418(AVZO-1418)
DB-1418 (AVZO-1418) is an EGFR/HER3 dual-targeting ADC based on topoisomerase 1 inhibitor, developed by Avenzo Therapeutics in collaboration with Duality Biotherapeutics. It is constructed using the unique Duality Innovative Bispecific Antibody Conjugate (DIBAC) platform of DualityBio. Currently, its global research and development has entered the phase I/II clinical trial stage. In July this year, Avenzo announced that in the 1st phase of the 1/2 phase clinical study evaluating AVZO-1418/DB-1418 for the treatment of patients with advanced solid tumors, the first patient has received the drug administration.
JS212
JS212 is a dual-antibody ADC targeting EGFR and HER3, developed by Junshi Biosciences. It is mainly used for the treatment of advanced malignant solid tumors. Its global research and development stage is also Phase I/II. Preclinical studies have shown that JS212 has a high affinity and specificity binding effect on EGFR and HER3, and has demonstrated significant tumor suppression effects in multiple animal models. At the same time, JS212 has good tolerability and safety.
Summary
EGFR and HER3 play significant roles in the occurrence, development, and resistance mechanism of non-small cell lung cancer. EGFR drives tumor proliferation, while HER3, as a key dimerization partner, strongly activates downstream signaling pathways such as PI3K/AKT. The functionally complementary heterodimer formed by these two proteins becomes an important target for overcoming EGFR-TKI resistance. The development of EGFR×HER3 bispecific drugs such as iza-bren has not only confirmed the scientific value of synergistic blockade but also brought new hope for prolonging patient survival. With the deepening of clinical research, dual-targeted therapy is expected to provide more effective and durable treatment options for patients with EGFR-mutated NSCLC.
References
Fang, W., et al. (2025, September 6–9). Updated efficacy and safety of BL-B01D1, a first-in-class EGFRxHER3 bispecific antibody-drug conjugate (ADC), in patients with previously treated EGFR-mutant non-small cell lung cancer (NSCLC) [Oral presentation]. 2025 World Conference on Lung Cancer, Barcelona, Spain. https://cattendee.abstractsonline.com/meeting/21151/Session/208
Zhou, F., et al. (2025, September 6–9). BL-B01D1 in combination with osimertinib in patients with untreated EGFR-mutant advanced NSCLC: Preliminary results from a phase II study [Oral presentation]. 2025 World Conference on Lung Cancer, Barcelona, Spain. https://cattendee.abstractsonline.com/meeting/21151/Session/208
Rosell, R., & Karachaliou, N. (2016). Large-scale screening for somatic mutations in lung cancer. The Lancet, 387(10026), 1354–1356. https://doi.org/10.1016/S0140-6736(16)30041-3
Campbell, M. R., Amin, D., & Moasser, M. M. (2010). HER3 comes of age: New insights into its functions and role in signaling, tumor biology, and cancer therapy. Clinical Cancer Research, 16(5), 1373–1383. https://doi.org/10.1158/1078-0432.CCR-09-1218
Engelman, J. A., & Jänne, P. A. (2008). Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non–small cell lung cancer. Clinical Cancer Research, 14(10), 2895–2899. https://doi.org/10.1158/1078-0432.CCR-07-2248
Amin, D. N., Campbell, M. R., & Moasser, M. M. (2010). The role of HER3 in the resistance of cancer cells to targeted therapies. Expert Opinion on Investigational Drugs, 19(Suppl. 1), S67–S78. https://doi.org/10.1517/13543781003624641
Schaefer, G., et al. (2011). A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell, 20(4), 472–486. https://doi.org/10.1016/j.ccr.2011.08.015
Zhang, H., et al. (2017). EGFR kinase domain mutations and their implications in targeted therapy for non-small cell lung cancer. Frontiers in Bioscience (Landmark Edition), 22(8), 1295–1306. https://doi.org/10.2741/4549
Leonetti, A., et al. (2019). Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. British Journal of Cancer, 121(9), 725–737. https://doi.org/10.1038/s41416-019-0573-8
Hanna, N., et al. (2017). Systemic therapy for stage IV non–small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline update. Journal of Clinical Oncology, 35(30), 3484–3515. https://doi.org/10.1200/JCO.2017.74.6065
Nakagawa, K., et al. (2023, October). Final progression-free survival results from the KEYNOTE-789 study of pembrolizumab plus chemotherapy versus chemotherapy for EGFR-mutant NSCLC [Conference presentation abstract LBA65]. ESMO Congress.
PR Newswire. (2023). First patient dosed in Phase 1/2 clinical study of novel EGFR/HER3 bispecific antibody-drug conjugate AVZO-1418. https://www.prnewswire.com/news-releases/first-patient-dosed-in-phase-12-clinical-study-of-novel-egfrher3-bispecific-antibody-drug-conjugate-avzo-1418db-1418-302500882.html
Bristol Myers Squibb. (2023). SystImmune and Bristol Myers Squibb announce a global strategic collaboration agreement for the development and commercialization of BL-B01D1. https://news.bms.com/news/details/2023/SystImmune-and-Bristol-Myers-Squibb-Announce-a-Global-Strategic-Collaboration-Agreement-for-the-Development-and-Commercialization-of-BL-B01D1/default.aspx
Avenzo Therapeutics & DualityBio. (2023). Avenzo Therapeutics and DualityBio announce exclusive global license for potential best-in-class EGFR/HER3 antibody-drug conjugate. https://avenzotx.com/press-releases/avenzo-therapeutics-and-dualitybio-announce-exclusive-global-license-for-potential-best-in-class-egfr-her3-antibody-drug-conjugate/
ClinicalTrials.gov. (2025). Study of BL-B01D1 in patients with EGFR-mutant NSCLC (NCT06888830). U.S. National Library of Medicine. https://clinicaltrials.gov/study/NCT06888830
ClinicalTrials.gov. (2025). Study of BL-B01D1 plus osimertinib in EGFR-mutant NSCLC (NCT07147348). U.S. National Library of Medicine. https://clinicaltrials.gov/study/NCT07147348
ClinicalTrials.gov. (2025). Study of AVZO-1418 in patients with advanced solid tumors (NCT06469008). U.S. National Library of Medicine. https://clinicaltrials.gov/study/NCT06469008
International Association for the Study of Lung Cancer (IASLC). (2025). IZA-BREN combination with osimertinib shows 100% response rate in EGFR-mutated NSCLC. https://www.iaslc.org/iaslc-news/press-release/iza-bren-combination-osimertinib-shows-100-response-rate-egfr-mutated














