In recent years, the pace of innovation in cancer immunotherapy has been accelerating, driven primarily by the influx of capital into the PD-1/VEGF bispecific antibody field. With the commercial validation of PD-1 monoclonal antibodies firmly established, investors and pharmaceutical companies have increasingly focused on innovative drugs that combine immune activation with anti-angiogenic mechanisms.
PD-1/VEGF bispecific antibodies, such as Ivonescimab from Summit Therapeutics, once sparked a wave of interest among global investors. At the end of last year, Merck and LaNova signed an exclusive licensing agreement for LM-299, with an upfront deal valued at nearly $600 million. Pfizer paid up to $1.25 billion upfront, along with potential milestone payments several times that amount, to secure rights to 3SBio’s SSGJ-707. Meanwhile, BioNTech and Bristol Myers Squibb launched a major collaboration on the PD-L1xVEGF bispecific antibody BNT327, with a total deal value exceeding $11 billion; the drug is currently in Phase III clinical trials. Learn more about our catalog.
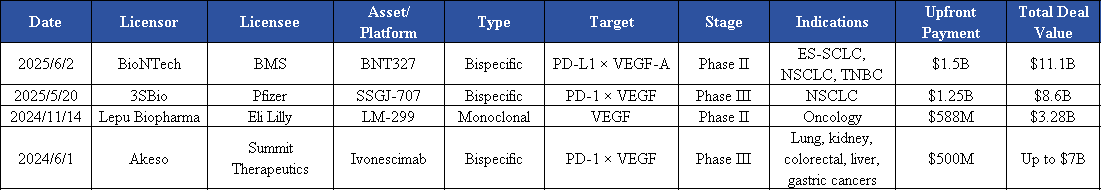
This surge of investment not only highlights the commercial potential of the field but also reflects the industry’s active exploration of higher-dimensional immunotherapy combination strategies amid increasingly intense competition.
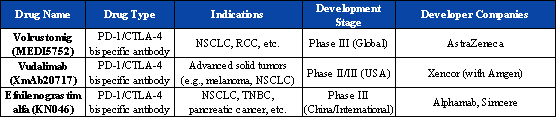
However, tracing the true starting point of the next stage in immunotherapy inevitably leads to the advent of PD-1/CTLA-4 bispecific antibodies. Unlike the PD-1/VEGF pathway, which represents a cross-mechanism parallel upgrade, PD-1/CTLA-4 stands as a hallmark achievement of the IO 2.0 era. Its core lies in the synergistic activation of dual immune checkpoints, which can simultaneously relieve tumor-induced immunosuppression and comprehensively enhance T cell function.
As early as 2015, Bristol Myers Squibb’s combination of nivolumab (PD-1) and ipilimumab (CTLA-4) had already established the clinical value of this approach in melanoma.
However, as bispecific antibodies gradually became a clinical norm, the industry quickly realized that simply combining two targets was no longer sufficient to simultaneously overcome the efficacy and tolerability ceiling. This prompted a shift in focus toward the IO 2.0+ era, where multi-target fusion strategies—exemplified by PD-1/VEGF/CTLA-4 trispecific antibodies—have emerged as a new frontier for research and investment. These approaches aim to simultaneously inhibit angiogenesis, relieve immunosuppression, and further enhance T cell activity, offering novel opportunities for resistant patient populations and a broader range of tumor types, while expanding the clinical landscape of immunotherapy.
As a result, the industry’s attention has moved toward IO 2.0+, more advanced therapies, with PD-1/VEGF/CTLA-4 trispecific antibodies becoming the new competitive high ground.
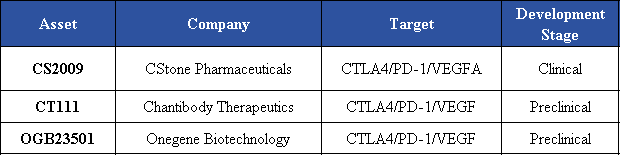
CS2009
CStone’s CS2009 is a pioneering candidate in the trispecific antibody field and has advanced to a global Phase I clinical trial, with the first patient dosed in Australia and plans to expand to China and the U.S. CS2009 is a trispecific molecule targeting PD-1, VEGFA, and CTLA-4, featuring a differentiated molecular design that leverages three synergistic mechanisms: activating tumor-infiltrating lymphocytes (TILs), relieving immune tolerance, and inhibiting tumor angiogenesis. Its therapeutic coverage spans multiple cancer types, including non-small cell lung cancer, liver cancer, gastric cancer, endometrial cancer, ovarian cancer, renal cell carcinoma, and cervical cancer, positioning it as a potential next-generation cornerstone IO therapy. It is expected to demonstrate superior efficacy compared with PD-1/VEGF and PD-1/CTLA-4 bispecific antibodies while maintaining a wider safety window.
Preclinical data indicate that CS2009 exhibits significantly stronger antitumor activity than potential competitors, including PD-1/CTLA-4 bispecifics, PD-1/VEGF bispecifics, and PD-1 plus anti-CTLA-4 combination therapies. In vitro studies show that CS2009 specifically activates tumor-infiltrating T cells and synergizes efficiently with VEGF blockade. In immunocompetent mouse models, CS2009 demonstrates superior tumor-killing effects compared with PD-1/CTLA-4 and PD-1/VEGF bispecifics. Toxicology studies indicate that CS2009 has a safety dose level notably higher than PD-1/CTLA-4 bispecifics and comparable to PD-1/VEGF bispecifics. These results suggest the potential to overcome treatment barriers in patients with low or negative PD-L1 expression.
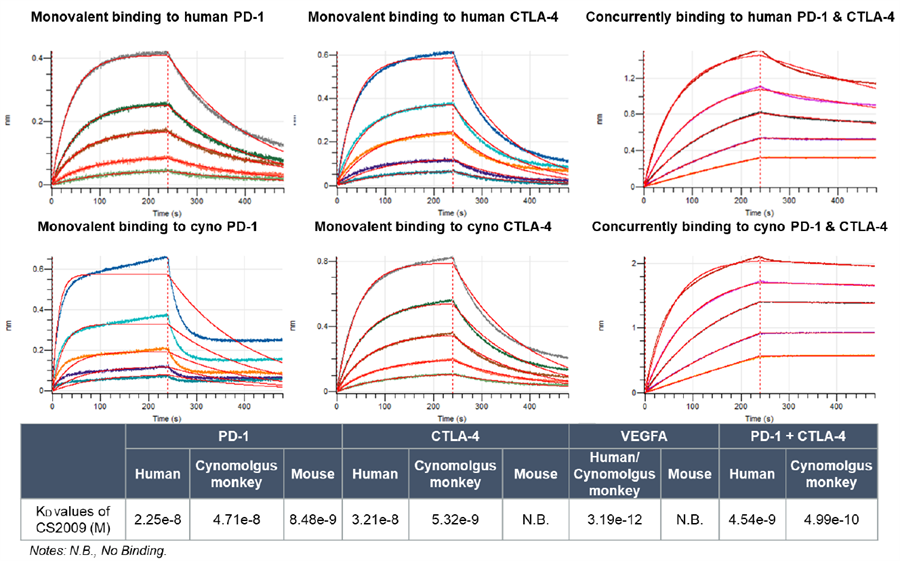
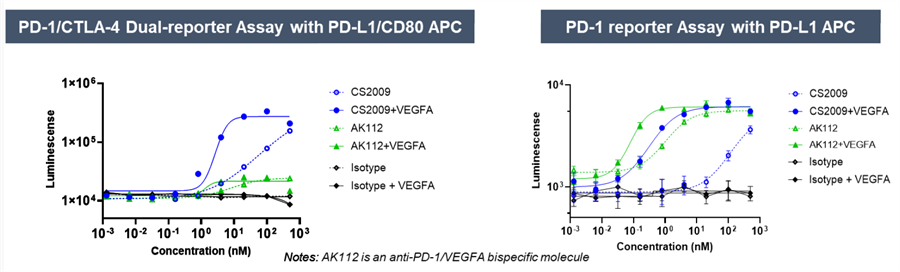
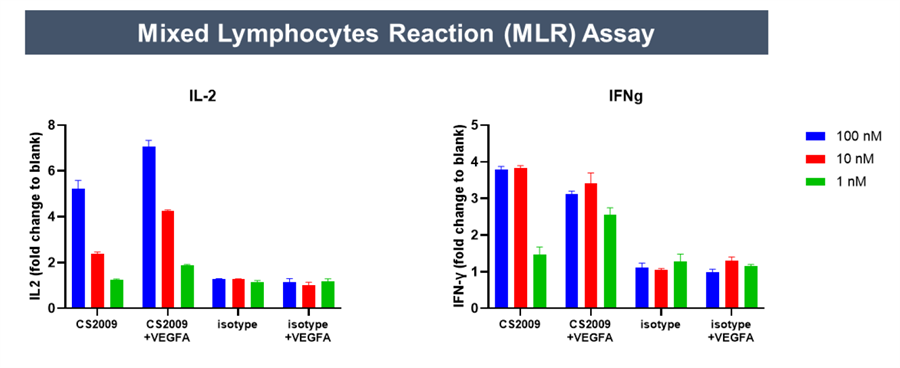
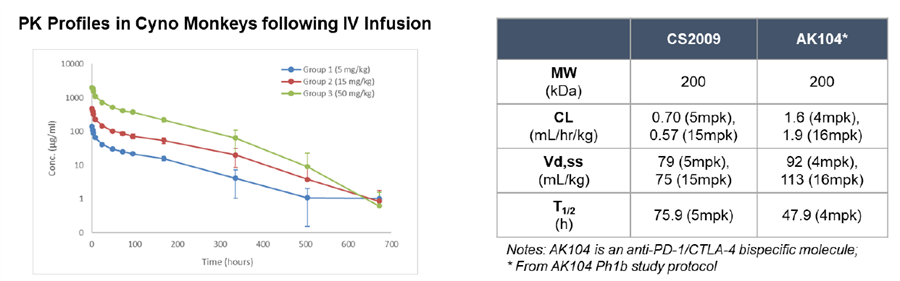
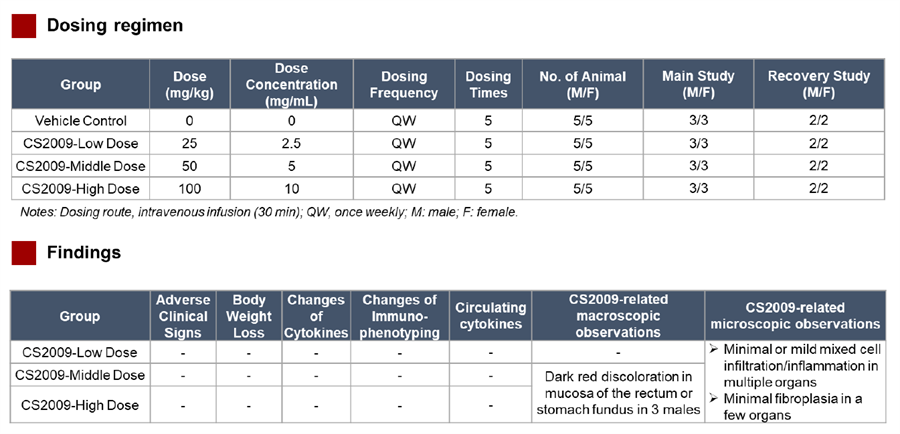
In March 2025, CStone Pharmaceuticals announced that its PD-L1/VEGF/CTLA-4 trispecific antibody CS2009 successfully dosed the first patient in Australia, marking the initiation of its global multicenter Phase I clinical trial. As of July 7, 2025, the global multicenter Phase I/II study of CS2009 is actively recruiting patients in Australia and China, with plans to expand to the U.S. for Phase II enrollment.
Positive expectations for the CS2009 trispecific product have significantly boosted investor sentiment toward CStone Pharmaceuticals, driving a continuous rise in its secondary market stock price and reflecting recognition of its innovative pipeline and global clinical strategy.
Conclusion
Overall, from the capital-driven surge in PD-1/VEGF bispecifics to the rapid rise of PD-1/VEGF/CTLA-4 trispecifics, immuno-oncology therapies are undergoing an upgrade from IO 2.0 to IO 2.0+. For companies, this represents not only a strategic choice in technological development but also a determinant of future market influence and competitiveness. Against the backdrop of concentrated investment and accelerated clinical progress, companies that are first to advance trispecific antibodies are well-positioned to become the next-generation industry leaders.
In this landscape, Genomeditech’s deep expertise in CTLA-4, PD-1, and VEGF targets, combined with its comprehensive cell line, antibody, and protein platforms, offers a systematic solution that covers the full drug discovery process. By providing high-quality tools for antibody screening, functional validation, and consistency evaluation, Genomeditech is fully enabling companies to accelerate their progression from research and development to clinical submission, reinforcing their competitive edge in the evolving immuno-oncology field.
Reference
BioNTech SE. (2025, June 2). BioNTech and Bristol Myers Squibb announce global strategic partnership to co-develop and co-commercialize next-generation bispecific antibody candidate BNT327 broadly for multiple solid tumor types. BioNTech. Retrieved from https://investors.biontech.de/news-releases/news-release-details/biontech-and-bristol-myers-squibb-announce-global-strategic
Pfizer Inc. (2025, May 19). Pfizer enters into exclusive licensing agreement with 3SBio. Pfizer. Retrieved from https://www.pfizer.com/news/press-release/press-release-detail/pfizer-enters-exclusive-licensing-agreement-3sbio
Merck & Co., Inc. (2024, November 14). Merck enters into exclusive global license for LM-299, an investigational anti-PD-1/VEGF bispecific antibody from LaNova Medicines Ltd. Merck. Retrieved from https://www.merck.com/news/merck-enters-into-exclusive-global-license-for-lm-299-an-investigational-anti-pd-1-vegf-bispecific-antibody-from-lanova-medicines-ltd/
Akeso, Inc. (2022, December 6). Akeso Inc. announces collaboration and license agreement for up to US$5 billion with Summit Therapeutics to accelerate global development and commercialization of its breakthrough bispecific antibody, Ivonescimab (PD-1/VEGF). PR Newswire. Retrieved from https://www.prnewswire.com/news-releases/akeso-inc-announces-collaboration-and-license-agreement-for-up-to-us5-billion-with-summit-therapeutics-to-accelerate-global-development-and-commercialization-of-its-breakthrough-bispecific-antibody-ivonescimab-pd-1vegf-301695787.html
Xencor, Inc. (2021). Xencor presents early clinical data from combination study of vudalimab. https://investors.xencor.com/news-releases/news-release-details/xencor-presents-early-clinical-data-combination-study-vudalimab
Alphamab Oncology. (2020). Alphamab Oncology initiates Phase III clinical trial for bispecific antibody KN046 in advanced solid tumors. https://www.alphamabonc.com/en/html/news/2126.html
AstraZeneca. (2023). AstraZeneca advances scientific leadership across multiple cancers with first data from four pivotal Phase III trials at ESMO. https://www.astrazeneca.com/media-centre/press-releases/2023/astrazeneca-advances-scientific-leadership-across-multiple-cancers-with-first-data-from-four-pivotal-phase-iii-trials-at-esmo.html
CStone Pharmaceuticals. (2025, March 3). CStone announces first patient dosed in global multicenter phase I clinical trial of CS2009, a PD-1/VEGF/CTLA-4 trispecific antibody. PR Newswire. https://www.prnewswire.com/news-releases/cstone-announces-first-patient-dosed-in-global-multicenter-phase-i-clinical-trial-of-cs2009-a-pd-1vegfctla-4-trispecific-antibody-302389842.html
Chantibody Therapeutics. (2025). AACR CT111. https://chantibody.com/aacr-ct111
OneGene Biotechnology. (n.d.). OGB23501. https://www.onegenebt.com/m33.php
BioNTech SE. (2025, June 2). BioNTech and Bristol Myers Squibb announce global strategic partnership to co-develop and co-commercialize next-generation bispecific antibody candidate BNT327 broadly for multiple solid tumor types. BioNTech. Retrieved from https://investors.biontech.de/news-releases/news-release-details/biontech-and-bristol-myers-squibb-announce-global-strategic
Pfizer Inc. (2025, May 19). Pfizer enters into exclusive licensing agreement with 3SBio. Pfizer. Retrieved from https://www.pfizer.com/news/press-release/press-release-detail/pfizer-enters-exclusive-licensing-agreement-3sbio
Merck & Co., Inc. (2024, November 14). Merck enters into exclusive global license for LM-299, an investigational anti-PD-1/VEGF bispecific antibody from LaNova Medicines Ltd. Merck. Retrieved from https://www.merck.com/news/merck-enters-into-exclusive-global-license-for-lm-299-an-investigational-anti-pd-1-vegf-bispecific-antibody-from-lanova-medicines-ltd/
Akeso, Inc. (2022, December 6). Akeso Inc. announces collaboration and license agreement for up to US$5 billion with Summit Therapeutics to accelerate global development and commercialization of its breakthrough bispecific antibody, Ivonescimab (PD-1/VEGF). PR Newswire. Retrieved from https://www.prnewswire.com/news-releases/akeso-inc-announces-collaboration-and-license-agreement-for-up-to-us5-billion-with-summit-therapeutics-to-accelerate-global-development-and-commercialization-of-its-breakthrough-bispecific-antibody-ivonescimab-pd-1vegf-301695787.html
Xencor, Inc. (2021). Xencor presents early clinical data from combination study of vudalimab. https://investors.xencor.com/news-releases/news-release-details/xencor-presents-early-clinical-data-combination-study-vudalimab
Alphamab Oncology. (2020). Alphamab Oncology initiates Phase III clinical trial for bispecific antibody KN046 in advanced solid tumors. https://www.alphamabonc.com/en/html/news/2126.html
AstraZeneca. (2023). AstraZeneca advances scientific leadership across multiple cancers with first data from four pivotal Phase III trials at ESMO. https://www.astrazeneca.com/media-centre/press-releases/2023/astrazeneca-advances-scientific-leadership-across-multiple-cancers-with-first-data-from-four-pivotal-phase-iii-trials-at-esmo.html
CStone Pharmaceuticals. (2025, March 3). CStone announces first patient dosed in global multicenter phase I clinical trial of CS2009, a PD-1/VEGF/CTLA-4 trispecific antibody. PR Newswire. https://www.prnewswire.com/news-releases/cstone-announces-first-patient-dosed-in-global-multicenter-phase-i-clinical-trial-of-cs2009-a-pd-1vegfctla-4-trispecific-antibody-302389842.html
Chantibody Therapeutics. (2025). AACR CT111. https://chantibody.com/aacr-ct111
OneGene Biotechnology. (n.d.). OGB23501. https://www.onegenebt.com/m33.php














