Immune checkpoint inhibitors (ICIs) can restore the function of exhausted cytotoxic T lymphocytes (CTLs) by blocking the PD-1/PD-L1 axis, achieving breakthrough progress in the treatment of various tumors. However, the efficacy of PD-1 inhibitors alone remains limited, as some tumors exhibit resistance, mainly due to restricted T cell proliferation and activation within the tumor microenvironment. To enhance antitumor immunity, cytokine engineering has emerged as a key strategy, with particular focus on modulating the interleukin-2 (IL-2) signaling pathway.
IL-2 is a key regulator of T cell proliferation, survival, and function, with its signaling primarily mediated through the high-affinity receptor IL-2Rαβγ (CD25/CD122/CD132). Although conventional high-dose IL-2 therapy can activate effector T cells, it also excessively stimulates regulatory T cells (Tregs), suppressing antitumor immunity and causing severe systemic toxicities, such as capillary leak syndrome (CLS). To address these issues, IL-2 variants (IL-2v) have been engineered to modulate IL-2’s binding affinity to CD25, selectively activating antitumor T cells while minimizing side effects.
Progress in PD-1 / IL-2 Drug Development
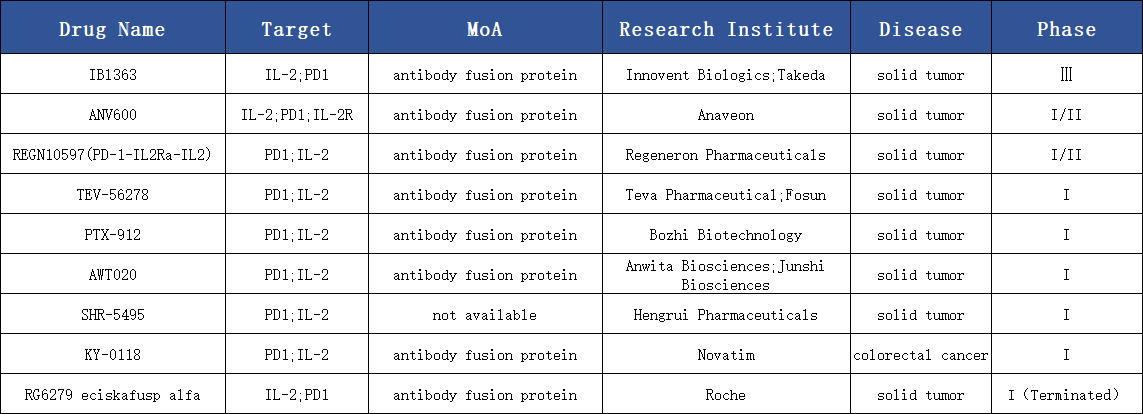
In the field of PD-1 × IL-2v fusion immunotherapy, two of the most prominent companies, Roche and Innovent Biologics, have proposed distinctly different molecular design concepts.Both companies employ a “cis-targeting” strategy, using the PD-1 antibody module to guide IL-2 delivery specifically to PD-1⁺ T cells, thereby achieving precise immune activation.
However, their approaches to IL-2 engineering diverge sharply: Roche’s PD-1×IL-2 fusion protein RG6279 utilizes a βγ-biased IL-2v, completely eliminating IL-2 binding to the α receptor (CD25) while retaining natural affinity for the βγ receptor. Through PD-1 antibody-mediated cis-targeting, IL-2v is delivered to PD-1⁺ T cells to bypass CD25 dependence and promote activation of stem-like CD8⁺ T cells (Tpex). Although this concept was initially published in Nature (2022) and OncoImmunology (2023), clinical results were disappointing; a similar drug, KY-0118, reported an ORR of 0% at the 2024 ASCO meeting, indicating limited efficacy.
In contrast, Innovent’s IBI363 adopts an α-biased IL-2v strategy, retaining high-affinity IL-2 binding to CD25 while substantially reducing βγ binding activity. This approach is based on research published in Nature Cancer (2023), which showed that tumor-specific CD8⁺ T cells (TSTs) co-express PD-1 and CD25. Retaining α binding more effectively activates PD-1⁺CD25⁺CD8⁺ TSTs, enhancing local immune responses. IBI363 reduces overall IL-2 activity, enabling safe delivery at high doses of 3 mg/kg to achieve effective PD-1 antibody-level treatment, while restoring high IL-2 activity within the tumor to elicit potent antitumor responses.
Therefore, Roche’s RG6279 resembles an early exploratory design, emphasizing structural experimentation and targeted delivery, whereas IBI363 embodies a second-generation IL-2 strategy, finely tuning IL-2R signaling to achieve more precise and effective antitumor immunity. The clinical outcomes of the two have also diverged sharply.
Roche vs. Innovent
Roche’s PD-1/IL-2v fusion protein RG6279 (Eciskafusp alfa) was officially discontinued in the second-quarter 2025 financial report, with the program terminated at Phase I. Although Roche continued to publish its preclinical research findings, it remained silent on clinical data, reflecting that the βγ-biased design may have fallen short of expected efficacy in practice.
In contrast, Innovent Biologics’ IBI363 has achieved significant success. In October 2025, Innovent entered a global strategic collaboration with Takeda Pharmaceutical, granting Takeda global rights to IBI363 outside the Greater China region. This partnership has been recognized within the industry as one of the most significant licensing deals in immuno-oncology in recent years. IBI363 is currently being evaluated for its therapeutic efficacy in non-small cell lung cancer (NSCLC) and microsatellite stable colorectal cancer (MSS CRC).
To date, IBI363 has demonstrated encouraging clinical activity in over 1,200 patients, showing notable efficacy especially in those previously resistant to PD-1 or IL-2 therapies. Thanks to its outstanding safety and efficacy profile, the drug has received FDA Fast Track designation for second-line squamous NSCLC. Moving forward, Innovent and Takeda will jointly advance its global development and commercialization in the U.S., further consolidating the strategic value of the PD-1–IL-2v combination in next-generation immunotherapy.
Conclusion
The development of PD-1 × IL-2v fusion immunotherapies illustrates how nuanced molecular design can dramatically influence both mechanistic outcomes and clinical success. Roche’s RG6279, with its βγ-biased IL-2v approach, represents an early exploratory strategy that prioritized structural innovation and targeted delivery but ultimately fell short in clinical efficacy. In contrast, Innovent’s IBI363 leverages an α-biased IL-2v design to selectively activate tumor-specific PD-1⁺CD25⁺ CD8⁺ T cells, achieving a balance between potent antitumor immunity and safety. Its promising clinical results, strategic global partnership with Takeda, and regulatory recognition underscore the potential of second-generation PD-1–IL-2v therapies to advance next-generation immuno-oncology. This contrast highlights the critical role of receptor-targeted cytokine engineering in shaping future immunotherapeutic strategies.
Given the significant differences between Roche and Innovent in the molecular design and clinical performance of PD-1 × IL-2v fusion proteins, scientists require an in vitro testing system that can simultaneously assess PD-1 binding capability and IL-2R signaling to better understand these differences and predict immune effects in vivo. In this context, Genomeditech’s H_CD25 CD122 CD132 Reporter Jurkat (hPD1 OE) Cell Line (GM-C41979) was developed, becoming an important tool for both product promotion and functional validation.
The H_CD25 CD122 CD132 Reporter Jurkat (hPD1 OE) cell line is a stable clonal Jurkat line constructed using lentiviral technology. It primarily expresses CD25, CD122, CD132, and PD-1, along with a signal-dependent luciferase reporter gene. When an anti-PD-1–IL-2v antibody binds PD-1 and delivers IL-2v to the IL-2 receptor, downstream IL-2R signaling induces luciferase expression. Luciferase readouts reflect pathway activation and can be used to evaluate the in vitro efficacy of relevant candidate drugs.
Know more at:
https://en.genomeditech.com/cell-line/H_CD25-CD122-CD132-Reporter-Jurkat%28hPD1-OE%29-Cell-Line
Reference
Anaveon. (2024). Anaveon announces IND approval of ANV600-001 (EXPAND) Phase I/II clinical study. Anaveon. https://anaveon.com/news/anaveon-announces-ind-approval-of-anv600-001-expand-phase-i-ii-clinical-study/
Regeneron Pharmaceuticals, Inc. (2025). Investigational pipeline. Regeneron. https://www.regeneron.com/science/investigational-pipeline
Teva Pharmaceutical Industries Ltd. (2025). Teva and Fosun Pharma enter into a strategic partnership to develop novel anti-PD1-IL2 therapy (TEV-56278) in immuno-oncology. Teva. https://www.tevausa.com/news-and-media/press-releases/teva-and-fosun-pharma-enter-into-a-strategic-partnership-to-develop-novel-anti-pd1-il2-therapy-tev-56278/
S. National Library of Medicine. (2025). A first-in-human (FIH), Phase I study of PTX-912 in patients with locally advanced/metastatic solid tumors. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT06190886
S. National Library of Medicine. (2025). Phase I clinical study of SHR-5495 in the treatment of advanced solid tumors. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT06059508
Novatim Pharmaceutical Co., Ltd. (n.d.). Novatim Pharmaceutical Co., Ltd. Novatim. http://en.novatim-zj.com/show-28-30-1.html
Roche Holding AG. (n.d.). Roche pipeline. Roche. https://www.roche.com/solutions/pipeline#2fed21d8-ce3e-4c4c-a9d0-519999c0984a
Fu, Y., Tang, R., & Zhao, X. (2023). Engineering cytokines for cancer immunotherapy: A systematic review. Frontiers in Immunology, 14, 1218082. https://doi.org/10.3389/fimmu.2023.1218082
Innovent Biologics. (2025). 2025 ASCO oral presentation: Innovent Biologics announces updated date of IBI363 (first-in-class PD-1/IL-2α-bias bispecific antibody fusion protein) from Phase 1 clinical studies in advanced colorectal cancer. PR Newswire. https://www.prnewswire.com/news-releases/2025-asco-oral-presentation-innovent-biologics-announces-updated-date-of-ibi363-first-in-class-pd-1il-2-bias-bispecific-antibody-fusion-protein-from-phase-1-clinical-studies-in-advanced-colorectal-cancer-302470217.html
Roche. (2025). Roche's Half-Year Results 2025 presentation. Roche Investor Relations. https://www.roche.com/investors/events/hy-2025
Takeda Pharmaceutical Company Limited. (2025, October 21). Takeda enters global strategic partnership with Innovent Biologics to bolster oncology pipeline with next-generation investigational medicines for treatment of solid tumors. Takeda Newsroom. https://www.takeda.com/newsroom/newsreleases/2025/innovent/
GuideChem. (2025, October 22). Roche terminates TIGIT trials, updates pipeline. GuideChem. https://www.guidechem.com/guideview/phar/roche-terminates-tigit-trials-updates-pipeline.html














