FOLR1 (FRα) is becoming an increasingly important focus in tumor therapy research, with multiple ADCs advancing rapidly in clinical development worldwide. Since October, Eli Lilly has registered the Phase III trial of LY4170156 (FRAmework‑01) for the treatment of platinum-resistant or platinum-sensitive ovarian cancer, while AstraZeneca has registered the Phase III trial of AZD5335 (TREVI‑OC‑01) targeting platinum-resistant ovarian cancer. In addition, several innovative FRα-targeted therapies developed by other companies, including Bio Thera and ProTarget Bio, are also progressing swiftly. These developments reflect the growing global clinical and R&D momentum for FRα as a tumor target, attracting significant attention from pharmaceutical companies worldwide.
Overview of Folate Receptor Alpha (FRα)
The folate receptor (FR) family is a group of membrane proteins associated with folate metabolism, primarily responsible for the high-affinity binding and internalization of folate. This family comprises four members—FRα, FRβ, FRγ, and FRδ—encoded by the FOLR1, FOLR2, FOLR3, and FOLR4 genes, respectively. FR family members display tissue-specific distribution in normal tissues, while they are often aberrantly overexpressed in tumor tissues, making them potential targets for cancer therapy and diagnostics.
Among them, FRα (encoded by FOLR1) is a GPI-anchored membrane protein, primarily localized at the apical surface of polarized epithelial cells in normal tissues such as the kidney, pancreas, lung, and ovarian epithelium. Its main function is to bind folate and its derivatives with high affinity and mediate their internalization, supporting DNA synthesis and cell proliferation.
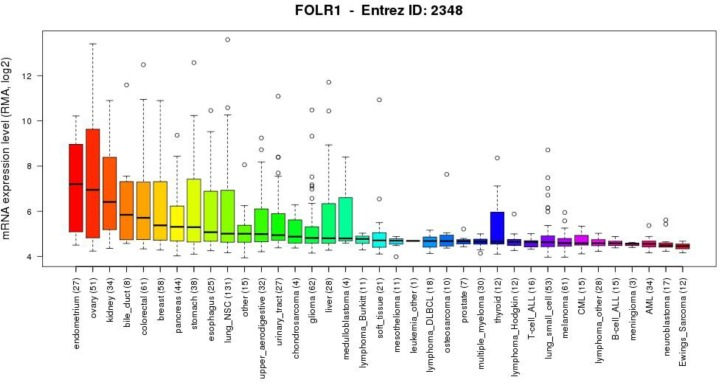
Expression of FRα
Compared with normal tissues, FRα is aberrantly overexpressed in various epithelial-derived cancers, including ovarian cancer, non-small cell lung cancer (NSCLC), breast cancer, and colorectal cancer, making it an ideal candidate target for drug delivery, immunotherapy, and diagnostics.
In ovarian cancer, 76–89% of epithelial ovarian tumors exhibit high FRα expression, which is closely associated with high-grade and advanced-stage disease. In NSCLC, 14–74% of cases show FRα overexpression, with membranous expression significantly higher in adenocarcinomas than in squamous cell carcinomas. In breast cancer, FRα is expressed in 35–68% of triple-negative breast cancers, while normal breast tissue shows minimal expression.
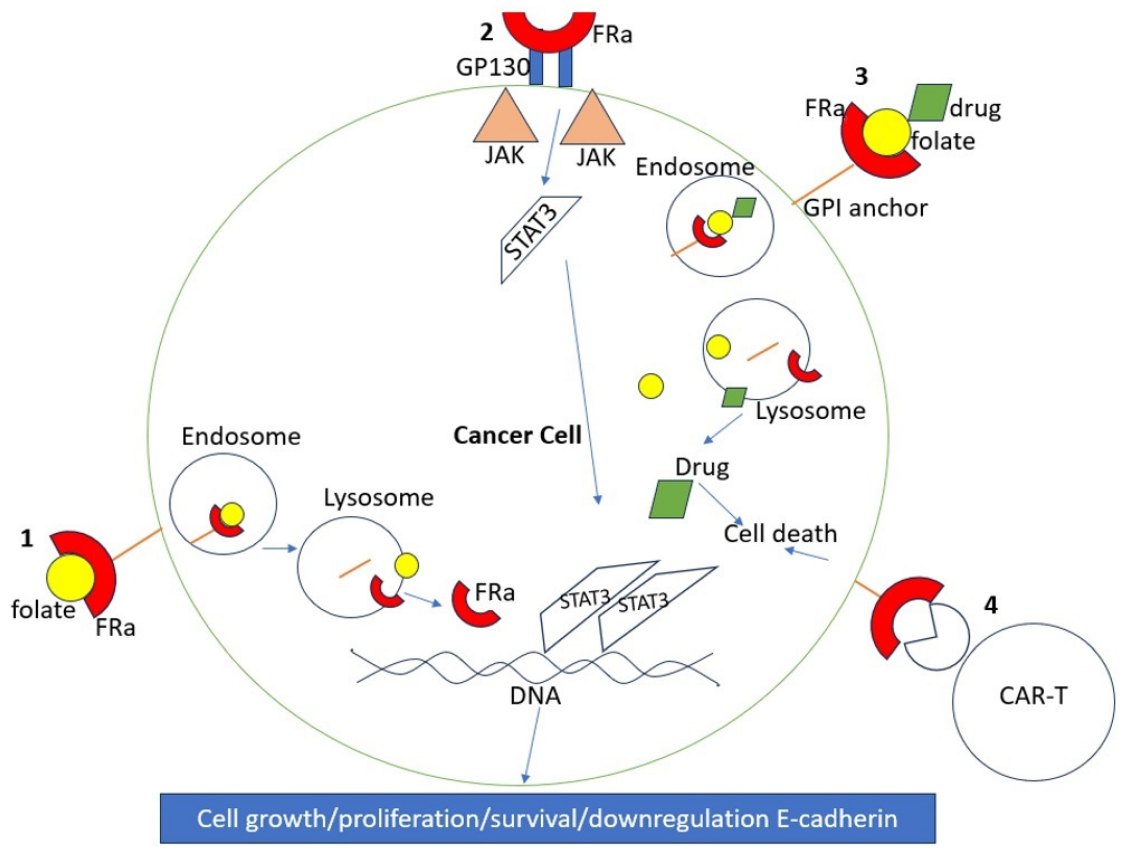
Physiological Functions of FRα
FRα functions primarily as a folate transporter, binding folate derivatives and mediating folate uptake and transport. It can also act as a transcription factor and plays a critical role in embryonic development, potentially mediating neural tube formation and maternal-fetal folate transport. Deficiency of FRα can lead to abnormal embryonic development and impaired organ function.
Beyond folate transport, FRα may influence tumor biology by activating downstream signaling pathways such as STAT3 and ERK1/2, thereby regulating tumor cell proliferation, migration, and the immune microenvironment, ultimately affecting tumor progression and therapy resistance. Some studies have also shown that high FRα expression is closely associated with clinical features such as tumor stage, invasiveness, and prognosis.
FRα-Targeted Therapeutics
Clinically, the high expression of FRα in malignant tumors makes it a potential target for anti-cancer drug development. Various strategies are currently being explored, including monoclonal antibodies, antibody-drug conjugates (ADCs), FRα-specific CAR-T cell therapies, vaccines, small-molecule drugs, and folate-drug conjugates.
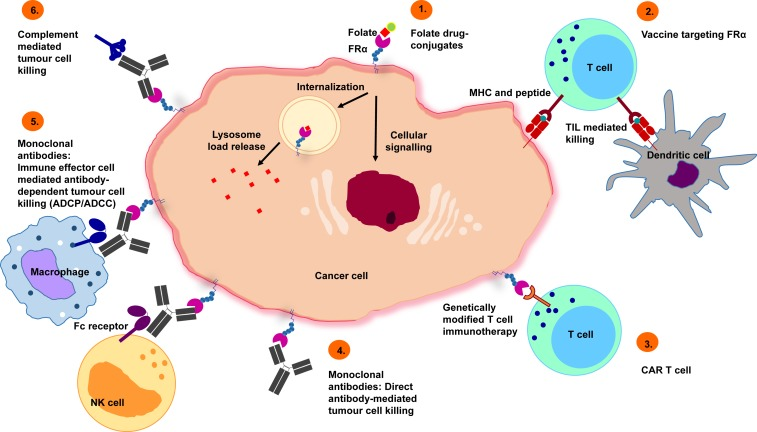
Antibody–Drug Conjugates (ADCs)
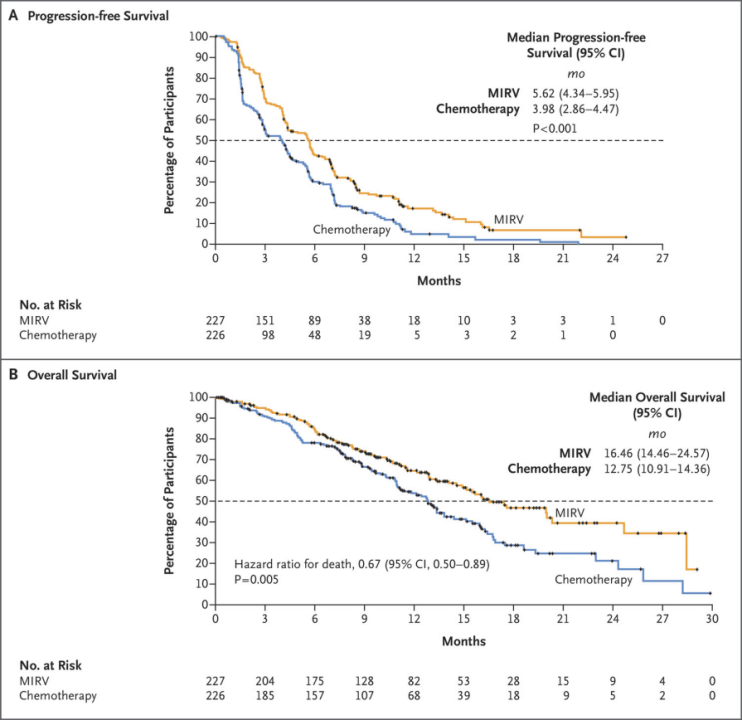
Among these strategies, ADCs have progressed the fastest. The first FRα-targeted ADC, Elahere, received accelerated approval from the FDA in 2022. Based on Phase III clinical data, it has demonstrated differentiated efficacy compared with chemotherapy in the PROC (platinum-resistant ovarian cancer) indication. As shown in the trial, the median progression-free survival (mPFS, INV) for the treatment group was 5.62 months, compared with 3.98 months in the chemotherapy control group. Overall survival (OS) was 16.46 months in the treatment group versus 12.75 months in the control group.
Huadong Medicine holds the rights to this marketed drug in China and also obtained NMPA approval in 2024.
In addition to the approved Elahere®, multiple FRα-targeted ADCs are advancing rapidly in development. Among them, BAT8006, PRO1184, LY4170156, and AZD5335 have entered Phase III clinical trials, reflecting a closely competitive development landscape following Elahere.
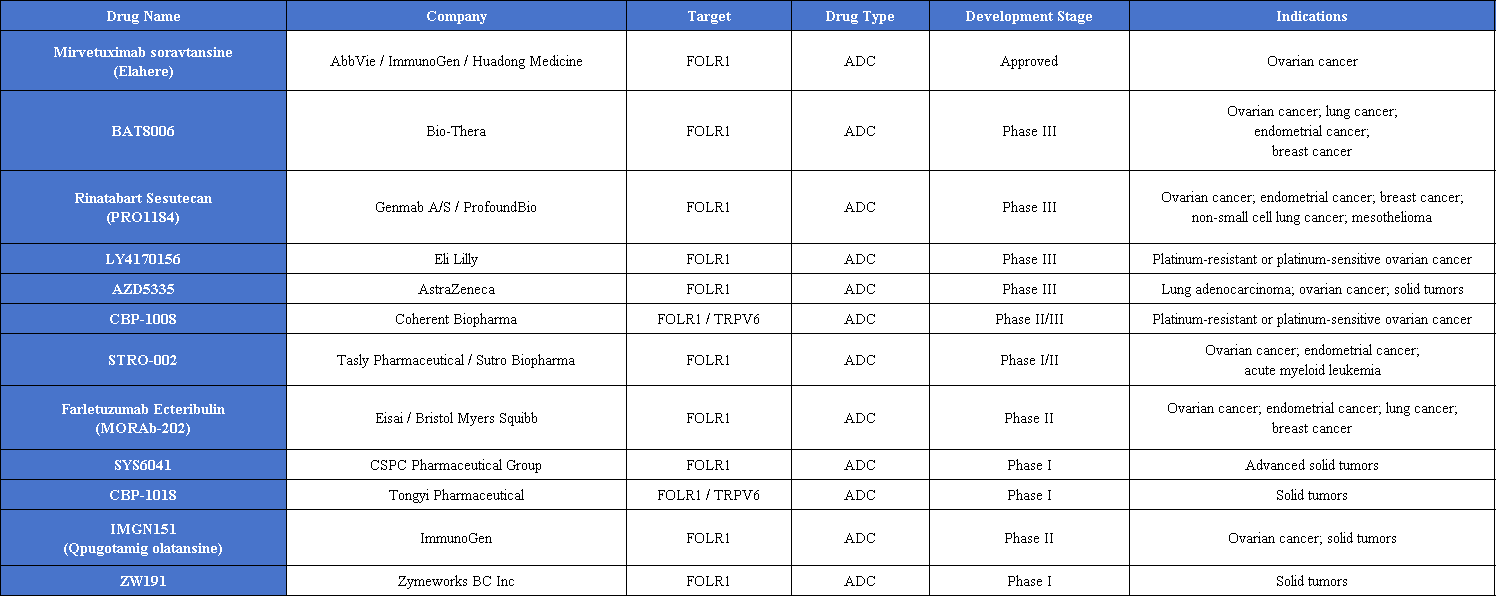
Monoclonal Antibodies
Monoclonal antibody drugs targeting FOLR1 include Farletuzumab, KHK2805, MOv19, MOv18-IgG1, and MOv18-IgE, among others.
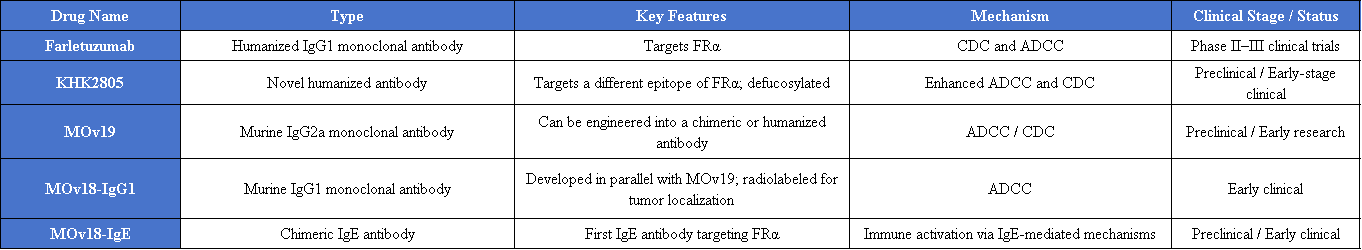
FRα-Specific CAR-T Cell Therapy
CAR-T cell therapies targeting FRα are also under investigation. A Phase I clinical trial involved engineering autologous T cells to express a chimeric antigen receptor (CAR) recognizing FRα, for the treatment of relapsed ovarian cancer. The CAR construct included a 4-1BB costimulatory domain. Although preliminary safety was confirmed, in vivo persistence and expansion of the T cells remained limited, and no significant tumor burden reduction was observed. Current research efforts are focused on improving T-cell durability, minimizing human anti-mouse antibody (HAMA) responses triggered by murine scFv domains, and optimizing strategies to enhance in vivo expansion.
Vaccine Therapy
Vaccine therapies targeting FRα are currently under development, including multi-epitope FRα peptide vaccines and dendritic cell (DC)–based vaccines. For example, a Phase II study is evaluating the efficacy of an FRα peptide vaccine combined with GM-CSF versus GM-CSF alone in patients with triple-negative breast cancer. Another Phase II study (FAROUT trial, NCT06639074) is investigating an FRα fusion–loaded DC vaccine in patients with stage III/IV ovarian, fallopian tube, or primary peritoneal cancer. Although still in early stages, these studies indicate that FRα-targeted vaccines have the potential to induce specific T-cell responses.
Small Molecules / Folate–Drug Conjugates (FDCs)
Among other FRα-related therapeutic strategies, folate-conjugated small molecules or folate–drug conjugates (FDCs) have also been extensively studied. For example, Vintafolide is an early folate–drug conjugate designed to deliver cytotoxic agents directly to tumor cells via FRα-mediated uptake; however, it did not meet its primary endpoint in Phase III clinical trials. On the other hand, small-molecule inhibitors such as BGC945 (also known as ONX0801) enter cells through FRα-mediated transport and inhibit thymidylate synthase, with partial clinical benefit observed in some ovarian cancer patients during Phase I studies. These approaches highlight the potential of exploiting FRα as a selective drug delivery pathway.
Conclusion
Overall, FRα (FOLR1) has emerged as a prominent target in the field of precision oncology, transitioning from early validation to multi-platform and multi-mechanism clinical translation. With the successful market approval of Elahere® and several follow-up ADC candidates entering pivotal studies, the clinical value of FRα-targeted therapies is being progressively validated. Looking ahead, the integration of diverse strategies—including ADCs, bispecific antibodies, CAR-T cells, and small-molecule conjugates—positions FRα as a promising breakthrough target for epithelial-derived tumors such as ovarian cancer, paving the way for more personalized approaches in cancer treatment.
References
AstraZeneca. (2023, April). AstraZeneca advances its pipeline and highlights progress in immuno-oncology, ADCs, cell therapy, and epigenetics at AACR. Retrieved from https://www.astrazeneca.com/media-centre/press-releases/2023/astrazeneca-advances-its-pipeline-and-highlights-progress-in-immuno-oncology-adcs-cell-therapy-and-epigenetics-at-aacr.html
Bio-Thera Solutions. (2024, May). Bio-Thera Solutions presents clinical data for BAT8006 (Folate Receptor Alpha ADC) at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting [Press release]. PR Newswire. https://www.prnewswire.com/news-releases/bio-thera-solutions-presents-clinical-data-for-bat8006-folate-receptor-alpha-adc-at-the-2024-american-society-of-clinical-oncology-asco-annual-meeting-302161233.html
ClinicalTrials.gov. (n.d.). A study of farletuzumab (MORAb-003) in subjects with platinum-sensitive ovarian cancer (NCT00849667). U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT00849667
ClinicalTrials.gov. (n.d.). Study of rina-tabat sesutecan (RLYB1234) in FRα-expressing ovarian cancer (NCT07213804). U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT07213804
ClinicalTrials.gov. (n.d.). STRO-002-GM1 in patients with advanced epithelial ovarian cancer (NCT04740398). U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT04740398
Eisai Co., Ltd. (2022, November 4). Eisai announces positive topline results for phase III MIRASOL study evaluating Elahere (mirvetuximab soravtansine-gynx) in FRα-high platinum-resistant ovarian cancer. https://www.eisai.com/news/2022/news202245.html
Elahere HCP. (n.d.). Elahere® (mirvetuximab soravtansine-gynx) for healthcare professionals. Retrieved from https://www.elaherehcp.com/
ImmunoGen. (2020, May 15). ImmunoGen to present preclinical data on IMGN151 at AACR Virtual Annual Meeting [Press release]. https://news.abbvie.com/2020-05-15-ImmunoGen-to-Present-Preclinical-Data-on-IMGN151-at-AACR-Virtual-Annual-Meeting
Koyama, S., et al. (2022). Folate receptor α (FRα) expression and clinical relevance in ovarian cancer. Frontiers in Oncology, 12, 974317. https://pmc.ncbi.nlm.nih.gov/articles/PMC9743174/
Mizuno, T., et al. (2018). Expression of folate receptor alpha is associated with favorable prognosis in ovarian clear cell carcinoma. Oncotarget, 9(58), 30087–30097. https://pubmed.ncbi.nlm.nih.gov/30068707/
Nature Communications. (2023). Targeting folate receptor alpha to enhance antibody–drug conjugate efficacy. Nature Communications, 14, Article 39679. https://www.nature.com/articles/s41467-023-39679-9
Sutro Biopharma. (n.d.). STRO-002-GM1: Folate receptor alpha–targeting antibody–drug conjugate. Retrieved from https://www.sutrobio.com/stro-002-gm1/
Targeted Oncology. (2025, January). FDA grants Fast Track Designation to rina-tabart sesutecan for FRα-expressing ovarian cancer. https://www.targetedonc.com/view/fda-grants-ftd-to-rinatabart-sesutecan-for-fr--expressing-ovarian-cancer
Zymeworks Inc. (2023, June). Zymeworks to present clinical data from Phase 1 trial of ZW191 antibody–drug conjugate. https://ir.zymeworks.com/news-releases/news-release-details/zymeworks-present-clinical-data-phase-1-trial-zw191-antibody/
AACR Journals. (2016). KHK2805: A novel ADCC and CDC enhanced antibody targeting folate receptor alpha [Abstract 2358]. Cancer Research, 76(14 Suppl). https://aacrjournals.org/cancerres/article/76/14_Supplement/2358/609574/
AACR Journals. (2025). SYS6041: A next-generation folate receptor alpha ADC with enhanced efficacy [Abstract 6832]. Cancer Research, 85(8 Suppl 1). https://aacrjournals.org/cancerres/article/85/8_Supplement_1/6832/760685/
ASCO. (2024). Abstract 378351: Clinical outcomes of FRα-targeted ADCs in ovarian cancer. ASCO Meeting Abstracts. https://www.asco.org/abstracts-presentations/ABSTRACT378351
Breast Cancer Clinical Trial. (n.d.). Multi-epitope folate receptor alpha peptide vaccine, GM-CSF, and cyclophosphamide in treating patients with triple negative breast cancer. SurvivorNet. Retrieved from https://www.survivornet.com/
Cheung, A., Bax, H. J., Josephs, D. H., Ilieva, K. M., Pellizzari, G., Opzoomer, J., Bloomfield, J., Fittall, M., Grigoriadis, A., Figini, M., Canevari, S., Spicer, J. F., Tutt, A. N., & Karagiannis, S. N. (2016). Targeting folate receptor alpha for cancer treatment. Oncotarget, 7(32), 52553–52574. https://doi.org/10.18632/oncotarget.9651
Chen, C. I., Li, W. S., Chen, H. P., Liu, K. W., Tsai, C. J., Hung, W. J., & Yang, C. C. (2022). High expression of folate receptor alpha (FOLR1) is associated with aggressive tumor behavior, poor response to chemoradiotherapy, and worse survival in rectal cancer. Technology in Cancer Research & Treatment, 21, 15330338221141795. https://doi.org/10.1177/15330338221141795
Food and Drug Administration. (2022, November 14). FDA grants accelerated approval to mirvetuximab soravtansine-gynx for FRα-positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer. U.S. Food and Drug Administration. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mirvetuximab-soravtansine-gynx-fra-positive-platinum-resistant
Gonzalez, T., Muminovic, M., Nano, O., & Vulfovich, M. (2024). Folate receptor alpha—A novel approach to cancer therapy. International Journal of Molecular Sciences, 25(2), 1046. https://doi.org/10.3390/ijms25021046
Kandalaft, L. E., Powell, D. J., Jr., & Coukos, G. (2012). A phase I clinical trial of adoptive transfer of folate receptor-alpha redirected autologous T cells for recurrent ovarian cancer. Journal of Translational Medicine, 10, 157. https://doi.org/10.1186/1479-5876-10-157
Mai, J., Wu, L., Yang, L., Sun, T., Liu, X., Yin, R., Jiang, Y., Li, J., & Li, Q. (2023). Therapeutic strategies targeting folate receptor α for ovarian cancer. Frontiers in Immunology, 14, 1254532. https://doi.org/10.3389/fimmu.2023.1254532
Massachusetts Medical Society. (2023). Mirvetuximab soravtansine in FRα-positive, platinum-resistant ovarian cancer. The New England Journal of Medicine, 389(22), 2075–2087. https://doi.org/10.1056/NEJMoa2309169
NCT06639074. (n.d.). Study of folate receptor alpha targeted therapy in solid tumors. ICH GCP Clinical Trials Registry. Retrieved from https://ichgcp.net/clinical-trials-registry/NCT06639074














