In 2025, Novartis made headlines with its $12 billion acquisition of Avidity Biosciences. The deal was driven by Avidity's unique AOC platform and promising pipeline. One of its leading candidates, AOC 1001 (del-desiran), delivers siRNA directly into muscle cells by targeting TfR1 (Transferrin Receptor 1), opening a new path for treating myotonic dystrophy. Other candidates in Avidity's portfolio also focus on rare skeletal muscle diseases, underscoring TfR1's strategic value in this field.
Overview of Transferrin Receptor 1
Iron is an essential trace element for life, participating in critical biological processes such as oxygen transport, energy metabolism, mitochondrial function, and DNA synthesis and repair. Transferrin receptor 1 (TfR1) serves as the primary pathway for cellular iron uptake, with most body iron transported through binding to transferrin (TF).
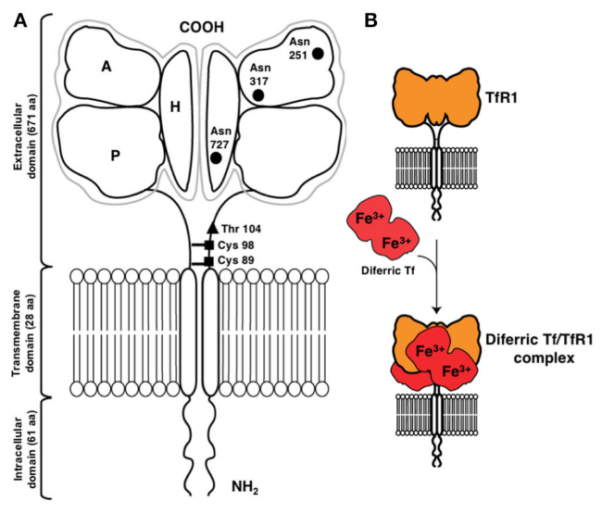
Also known as CD71, TfR1 is a 90 kDa type II transmembrane glycoprotein composed of 760 amino acids. It exists as a homodimer (180 kDa), with two monomers connected by extracellular disulfide bonds. Its structure comprises three main regions: a short intracellular N-terminal domain, a single transmembrane helix, and a large extracellular C-terminal domain. The extracellular C-terminal domain is the functional core, containing high-affinity binding sites for transferrin. TfR1 mediates iron uptake by binding to iron-loaded transferrin and internalizing it via receptor-mediated endocytosis, maintaining intracellular iron homeostasis and metabolic balance.
There are two types of transferrin receptors: TfR1 and TfR2, which are similar in structure and function. TfR1 is widely expressed in most nucleated cells and binds transferrin with higher affinity, making it the major iron entry route. In contrast, TfR2 has lower transferrin affinity, lacks iron-responsive elements, and is primarily expressed in hepatocytes.
Distribution and Expression of TfR1
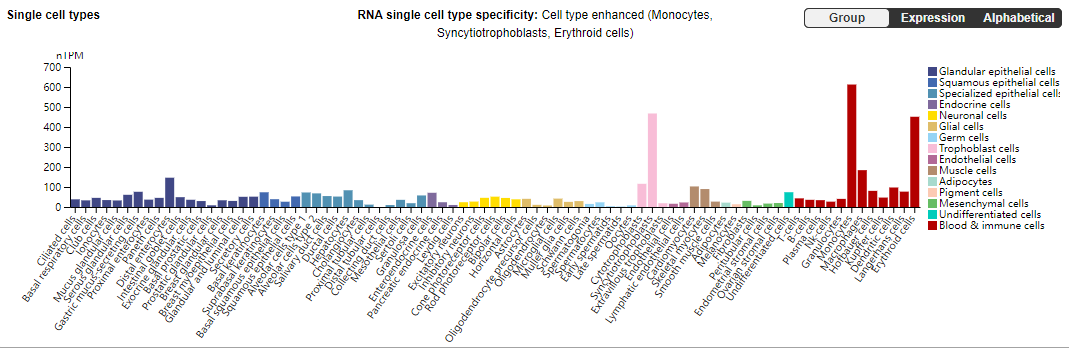
TfR1 is widely expressed across various tissues and cell types, including the immune system, hematopoietic system such as bone marrow stem cells, red blood cells, and white blood cells, nervous system including neurons and glial cells, reproductive system, heart, liver, and kidneys. These cells rely on TfR1-mediated iron uptake to meet the iron demands necessary for metabolic activity, differentiation, and growth.
TfR1 expression is influenced by multiple factors, including intracellular iron levels, cell differentiation status, hormonal regulation, and inflammatory conditions. When intracellular iron is low, TfR1 expression is upregulated to increase iron uptake; conversely, when iron is sufficient, its expression is suppressed. This feedback regulation underscores TfR1’s central role in iron transport and metabolism in the human body.
Overview of Disease Therapy and Drug Development Targeting TfR1
Beyond its key role in iron metabolism, TfR1 (CD71) has attracted increasing attention due to its abnormal expression and pathological involvement in cancer and neurodegenerative diseases.
TfR1 and Cancer
Malignant cells often exhibit a high dependency on iron and show significantly elevated surface expression of TfR1. Its extracellular accessibility, internalization capability, and central role in cancer cell pathology make TfR1 an ideal target for antibody-mediated therapy.
Cancer therapies targeting TfR1 can be achieved through two main approaches. The first, widely adopted, is indirect drug delivery via transferrin, ferritin, or specific TfR1 antibodies. This approach enables targeted delivery of proteins such as toxins, nucleic acids such as oligonucleotides, and viral vectors. Additionally, TfR1-targeted nanomedicines and other formulations, including ADCs (antibody-drug conjugates) or PDCs (peptide-drug conjugates), can further enhance targeting specificity and therapeutic efficacy.
The second approach is direct antibody-based therapy, where antibodies inhibit TfR1 function and/or engage antibody-mediated effector mechanisms, including antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC), to exert anticancer effects.
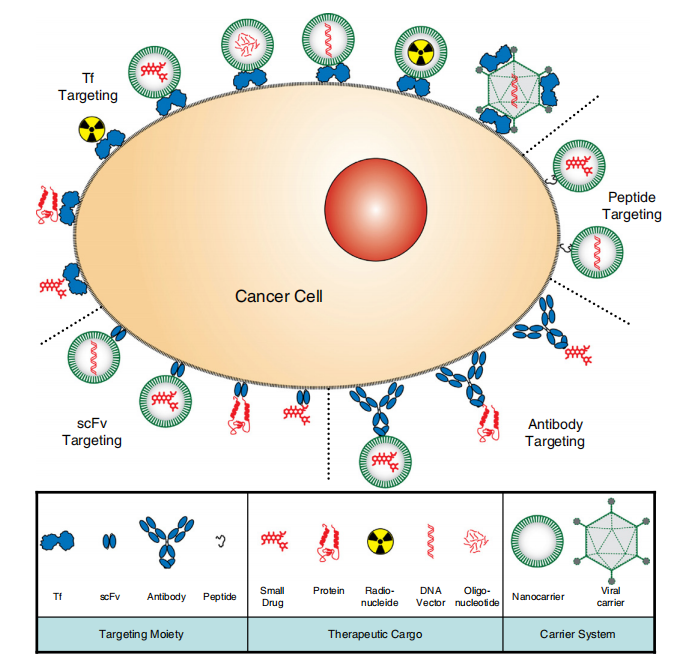
It is also important to note that these two approaches to TfR1-targeted cancer therapy are not mutually exclusive. Antibodies with direct cytotoxicity can also be used for drug delivery.
TfR1 and Neurodegenerative Diseases
In recent years, TfR1-based targeted therapies have been rapidly advancing. Some studies leverage TfR1 to enhance antibody transport across the blood-brain barrier, combining it with anti-β-amyloid antibodies to form specific bispecific antibodies, improving treatment outcomes for Alzheimer’s disease.
For example, Roche’s investigational drug Trontinemab is a bispecific antibody targeting Aβ and TfR1, developed for the treatment of Alzheimer’s disease. In the Phase Ib/IIa Brainshuttle™ AD study, the latest clinical data showed that treatment with Trontinemab at a dose of 3.6 mg/kg rapidly and substantially reduced amyloid levels. After 28 weeks, 91% of participants fell below the PET-positive threshold for amyloid, and 72% achieved amyloid levels ≤11 CL. Trontinemab also demonstrated a favorable safety and tolerability profile.
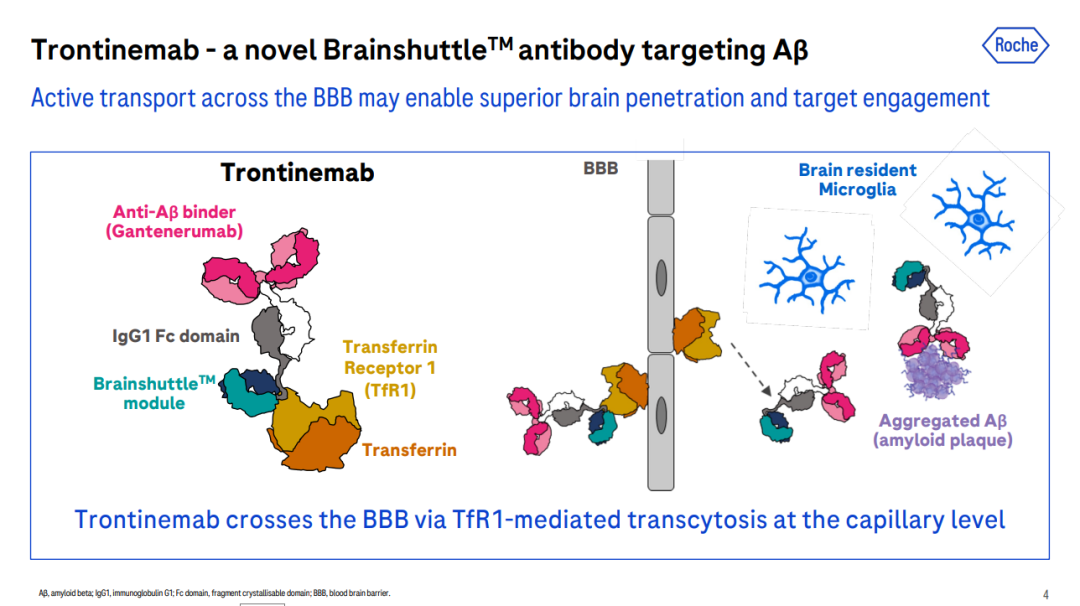
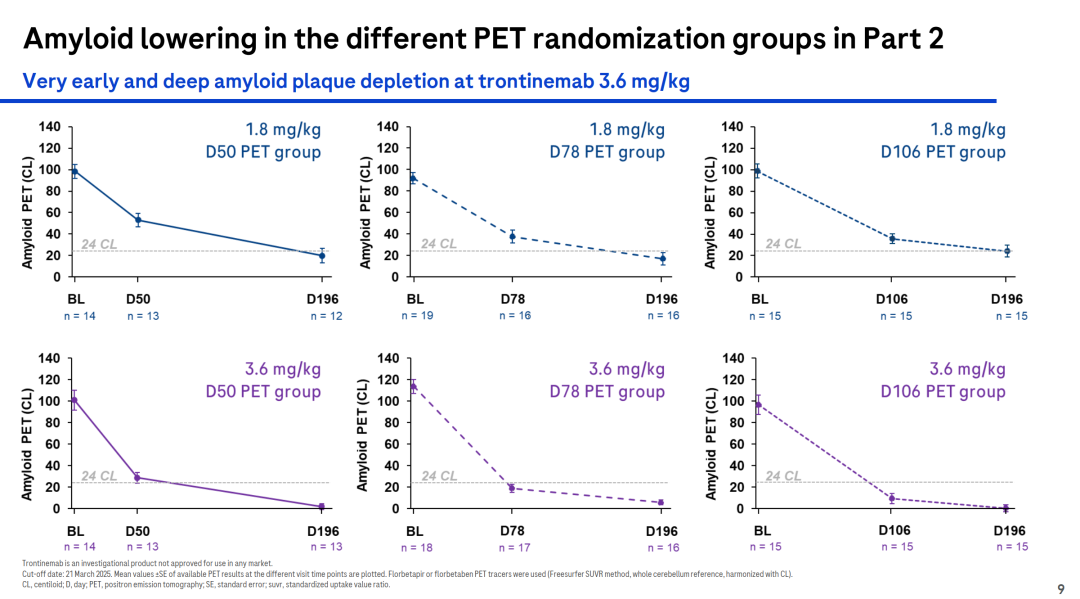
Current Status of Clinical Development for TfR1-Targeted Drugs
Given the pivotal role of TfR1 in iron metabolism and neurodegenerative diseases, therapeutic strategies targeting TfR1 have gained significant importance.
At present, multiple TfR1-targeted drugs are under clinical development, encompassing monoclonal antibodies, bispecific antibodies, fusion proteins, and antibody-drug conjugates (ADCs). These candidates are being investigated across a broad range of indications, including anemia, iron metabolism disorders, infectious diseases, neurodegenerative diseases, and cancer.
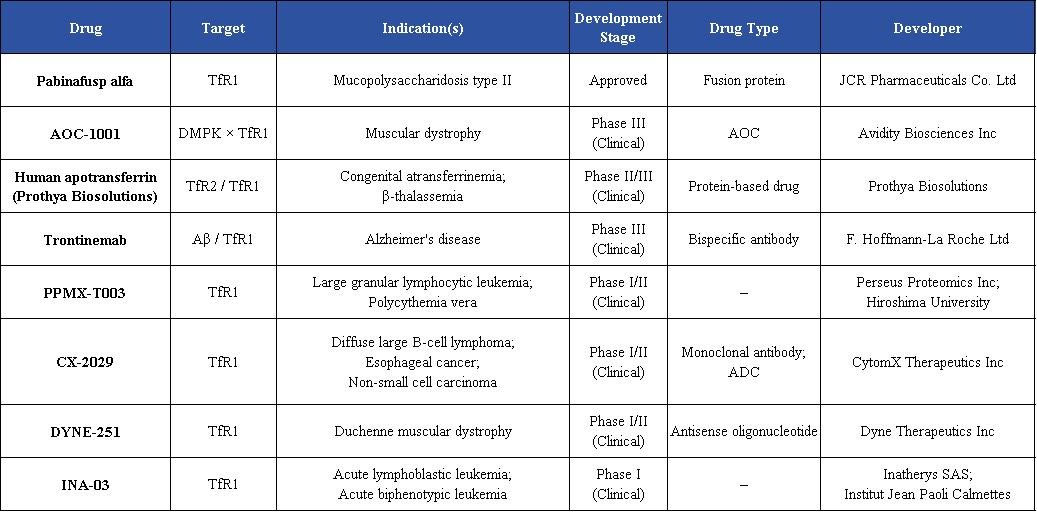
Conclusion
As a central regulator of cellular iron metabolism, TfR1 is essential for normal physiological processes. In tumor cells, its expression is often markedly elevated to meet the high iron demand required for rapid proliferation. This characteristic makes TfR1 an important molecular entry point for targeted drug delivery, antibody-drug conjugates (ADCs), and siRNA conjugate therapies, highlighting its vast potential in clinical applications and translational research.
References
Avidity Biosciences. (n.d.). DM1: Myotonic dystrophy type 1 program. Avidity Biosciences. https://www.aviditybiosciences.com/pipeline/dm1
Bloomberg News. (2025, July 13). China’s drugmakers are catching up to U.S. Big Pharma with new medicine innovation. Bloomberg. https://www.bloomberg.com/news/features/2025-07-13/china-drugmakers-catching-up-to-us-big-pharma-with-new-medicine-innovation
Candelaria, J., et al. (2021). Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents. Frontiers in Immunology, 12, 607692. https://doi.org/10.3389/fimmu.2021.607692
CytomX Therapeutics. (n.d.). CX-2029: A Probody drug conjugate targeting CD71 (transferrin receptor): Results from a first-in-human study (PROCLAIM-CX-2029) in patients with advanced cancer. CytomX Therapeutics. https://cytomx.com/cx-2029-a-probody-drug-conjugate-targeting-cd71-transferrin-receptor-results-from-a-first-in-human-study-proclaim-cx-2029-in-patients-with-advanced-cancer/
Dyne Therapeutics. (n.d.). Dyne-251 for Duchenne muscular dystrophy (DMD). Dyne Therapeutics. https://www.dyne-tx.com/dyne-251-for-dmd/
Inatherys. (n.d.). About Inatherys. https://www.inatherys.com/#about
JCR Pharmaceuticals. (2025, February). JCR Pharmaceuticals presents long-term clinical data on pabinafusp alfa for the treatment of mucopolysaccharidosis type II (MPS II) at ICIEM 2025. JCR Pharmaceuticals Newsroom. https://jcrpharm.com/jcr-pharmaceuticals-presents-long-term-clinical-data-on-pabinafusp-alfa-for-the-treatment-of-mucopolysaccharidosis-type-ii-mps-ii-at-iciem-2025/
Li, Y., et al. (2022). TfR1 mediated iron uptake regulates bone mass in mice via osteoclast mitochondria and cytoskeleton. eLife, 11, e73539. https://doi.org/10.7554/eLife.73539
PPMX Therapeutics. (n.d.). Pipeline. PPMX Therapeutics. https://www.ppmx.com/en/rd/pipeline/
Roche. (2025, July 28). Roche provides business update and reports solid results for the first half of 2025. Roche Media Releases. https://www.roche.com/media/releases/med-cor-2025-07-28
Roche Medical. (2023). The anti-amyloid beta antibody presentation (CTAD 2023) [PDF]. https://medically.roche.com/global/en/neuroscience/ctad-2023/medical-material/CTAD-2023-presentation-kulic-the-anti-amyloid-beta-pdf.html
Shen, Y., Li, X., Dong, D., Zhang, B., Xue, Y., & Shang, P. (2018). Transferrin receptor 1 in cancer: A new sight for cancer therapy. American Journal of Cancer Research, 8(6), 916–931.
UniProt. (n.d.). Transferrin receptor protein 1 (TfR1). https://www.uniprot.org
Wang, G., et al. (2017). Transferrin receptor 1 (TfR1) in cancer: A new sight for cancer therapy. Tumour Biology, 39(11), 1010428317732741. https://doi.org/10.1177/1010428317732741