In the high-priced bidding battle between Pfizer and Novo Nordisk for Metsera’s GLP-1 assets, one previously low-profile target has moved into the spotlight: amylin.
This target, considered an important direction for the next stage of weight-loss drug development, has significantly increased the strategic value of the acquisition.
Key Focus: Amylin
Amylin is a 37–amino-acid peptide hormone stored in pancreatic β-cells. When nutrients enter the small intestine, amylin is secreted into the bloodstream together with insulin, and its levels rise and fall in parallel with insulin. It helps regulate food intake by increasing the feeling of fullness and reducing energy consumption. Additionally, amylin promotes energy metabolism and reduces adipose tissue mass, contributing to the regulation of body weight and blood glucose.
The metabolic effects of amylin are primarily mediated through amylin receptors (AMYRs), which are complexes of the calcitonin receptor (CALCR/CTR) and receptor activity-modifying proteins (RAMPs). Activation of AMYRs slows gastric emptying, enhances satiety, suppresses appetite, lowers glucagon levels, and improves leptin signaling and glucose metabolism.
Compared with GLP-1–based drugs, amylin can significantly improve muscle retention and reduce muscle loss during weight loss, achieving a “fat loss with minimal muscle loss” effect, while also showing better gastrointestinal tolerability. This mechanism makes the AMY/CALCR target an important drug target for the treatment of diabetes and obesity and provides a scientific basis for next-generation weight-loss and metabolic therapies.
Returning to the core of the acquisition—Metsera:
The company has a limited pipeline, with only three candidates—MET-097i, MET-233i, and MET-002—currently in clinical development, while the remaining programs are still at the preclinical stage. These three clinical-stage programs are therefore the main focus of all competition.
Among them, MET-097i (a biased GLP-1RA activator) and its oral version MET-002 remain iterative products along the GLP-1 pathway. In terms of mechanism, positioning, and market potential, they are unlikely to move beyond the highly competitive GLP-1 landscape. Therefore, although they contribute to the investment narrative, they are not the decisive assets in this high-priced acquisition battle.
What has truly drawn fierce competition among the industry giants is MET-233i—a highly innovative long-acting amylin analog.
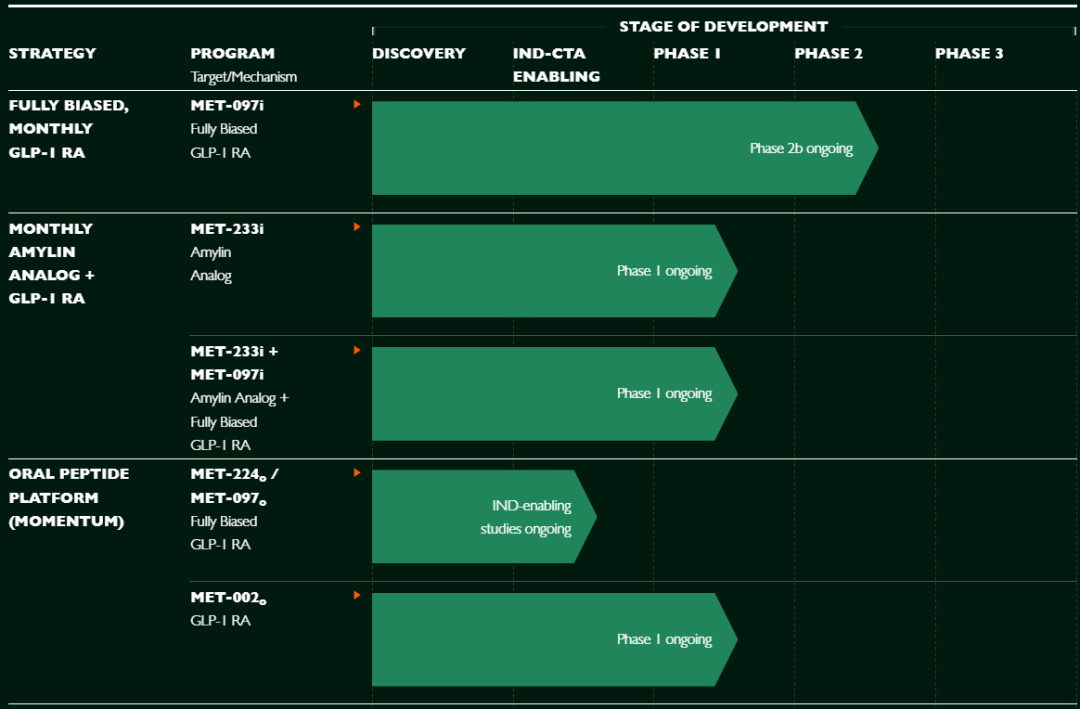
MET-233i is an ultra-long-acting monthly formulation amylin analog. Although it is currently only in Phase 1 clinical trials, its potential has already generated expectations for the “next-generation weight-loss target,” making it Metsera’s core competitive asset.
Clinical data show that MET-233i achieved up to 8.4% weight reduction over 36 days. While this is lower than some competitors, it has a half-life of 19 days, enabling once-monthly dosing, which is extremely rare among similar products. This combination of efficacy and low-frequency dosing represents the most scarce value in the current weight-loss therapy landscape. Metsera is conducting two early-stage studies, using MET-233i either as a monotherapy or in combination with MET-097i. Currently, Phase 1 trials are underway for MET-233i as a single agent, combined with MET-097i, and in an oral formulation.
Amylin can act synergistically with GLP-1 to enhance weight-loss effects. Metsera has initiated combination trials of MET-233i with the GLP-1 receptor agonist MET-097i, aiming for once-monthly dosing with matched half-lives and cycles, optimizing both efficacy and convenience. This combination is viewed by the market as a new direction in weight-loss therapy, offering significant potential. A similar mechanism has been validated in Novo Nordisk’s CagriSema (semaglutide + cagrilintide) combination, where Phase 3 trials showed a 22.7% weight reduction. Although it did not meet the preset target, it achieved efficacy comparable to tirzepatide, demonstrating the value of amylin as a synergistic target.
This suggests that if GLP-1 represents the “first phase” of weight-loss therapy, amylin is likely to drive the “second phase” of the industry. The logic mirrors the search for new incremental targets after PD-1/PD-L1, such as TIGIT and OX40—pursuing new opportunities rather than competing within existing targets.
Pfizer’s willingness to invest heavily in Metsera essentially reflects a strategic entry into the post–GLP-1 era. Within Pfizer’s relatively weaker GLP-1 portfolio, MET-233i carries far greater strategic value than it appears.
Amylin: The Next Growth Driver Beyond GLP-1
As one of the most closely watched targets in the weight-loss field, the amylin pathway has seen a surge of business development deals. In March this year, Roche and Zealand reached a collaboration agreement for petrelintide with a $1.4 billion upfront payment and a total deal value of $5.3 billion. AbbVie also invested $2.2 billion to acquire Denmark-based Gubra, strengthening its portfolio with the long-acting amylin analog GUB014295—marking AbbVie’s first entry into the amylin-based weight-loss market.
Across the industry, an increasing number of pharmaceutical companies are viewing amylin as the next growth driver beyond GLP-1, with giants such as Novo Nordisk, Eli Lilly, and Roche actively competing to establish their presence in this space.
Novo Nordisk Advances in Multi-Target Weight-Loss Therapies
lCagrisema (GLP-1 + Amylin combination): Phase 3 trials have been successful, showing approximately 23% weight reduction after 68 weeks, outperforming semaglutide monotherapy by 7%.
lAmycretin (GLP-1/Amylin dual agonist): Early clinical studies indicate strong weight-loss potential. Treatment with 60 mg/week for 36 weeks resulted in 24.3% weight reduction. Novo Nordisk is actively advancing Phase 3 trials for both subcutaneous and oral formulations of Amycretin.
lNNC0662-0419 (GLP-1/GIP/Amylin triple agonist): In September this year, Novo Nordisk registered a Phase 2 weight-loss trial on ClinicalTrials.gov, marking a further strategic expansion into multi-target co-agonist therapies.
Eli Lilly (biased Amylin agonist): Preliminary clinical data for Eloralintide, presented at the ADA conference, showed an average 20.1% weight reduction over 48 weeks in the highest dose group. Phase 3 trials are planned. These results reinforce Eli Lilly’s leadership in the weight-loss market and highlight the accelerating shift from single-target to multi-target synergistic therapies.
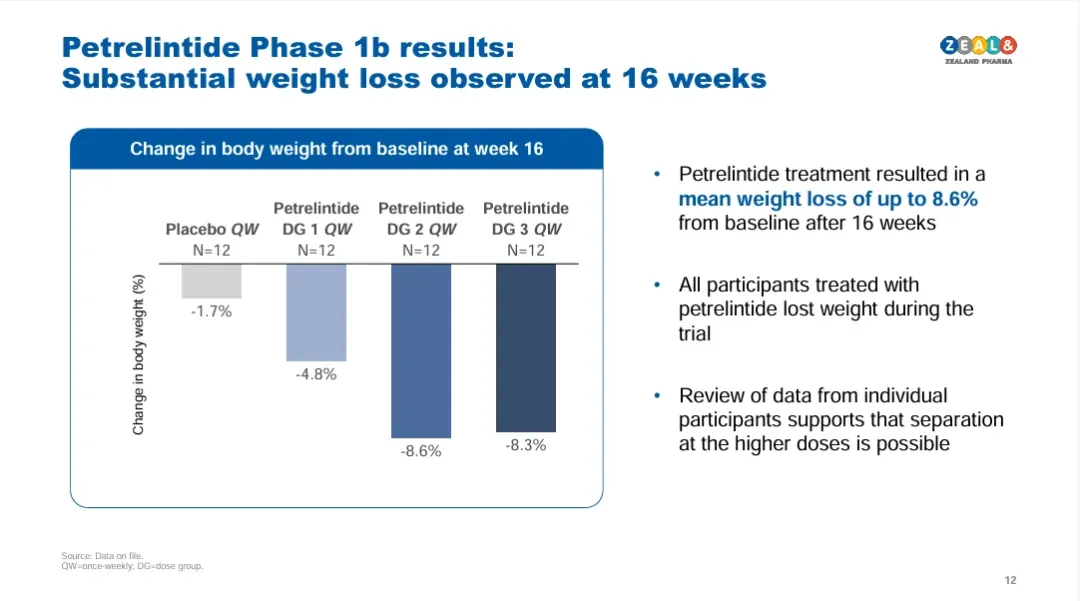
Petrelintide: Early data indicate 8.6% weight reduction over four months with a favorable gastrointestinal tolerability profile.
GUB014295 is currently in Phase 1 clinical trials. In the single ascending dose (SAD) study, GUB014295 showed good tolerability and favorable pharmacokinetic properties, with a half-life of 11 days, supporting the potential for once-every-two-weeks dosing. Even a single dose produced dose-dependent weight reduction, with the high-dose group (3.5–6.0 mg) showing an average 3% body weight loss.
AstraZeneca is also advancing early clinical development of its amylin program.
Chinese companies are accelerating their development of amylin therapies, with BrightGene Bio (BG1812) being the most advanced. Preclinical data show that BG1812 induces significant weight loss in diet-induced obese rat models and demonstrates synergistic effects when combined with BG0504 (GLP-1/GIP agonist). On November 4, BrightGene registered a Phase 1 clinical trial in the U.S. for the amylin analog BGM1812 on ClinicalTrials.gov, entering the global competitive landscape.
Summary: Amylin as a Key Industry Target
Based on mechanism, clinical potential, and investment activity, amylin is emerging as an important strategic target in the weight-loss drug field. The Metsera acquisition reflects the industry’s focus on amylin and the development of therapies beyond GLP-1.
The M&A activity is only the initial stage, and further competition in weight-loss drug development is ongoing.
Reference
ClinicalTrials.gov. (n.d.). Study of pramlintide in obesity (NCT00229658). U.S. National Library of Medicine. https://clinicaltrials.gov/study/NCT00229658
BioSpace. (2025, March 3). Novo Nordisk presents phase 3 data for next-generation amylin cagrilintide, leading to advancement into dedicated clinical programme. https://www.biospace.com/press-releases/novo-nordisk-presents-phase-3-data-for-next-generation-amylin-cagrilintide-leading-to-advancement-into-dedicated-clinical-programme
Novo Nordisk. (n.d.). R&D pipeline. https://www.novonordisk.com/science-and-technology/r-d-pipeline.html
Novo Nordisk Trials. (n.d.). A research study on how a dose of NNC0662-0419 works in Japanese, Chinese and non-Asian participants living with overweight or obesity. https://www.novonordisk-trials.com/trials-conditions/all-trials-v2/NN9662-8159.html
Eli Lilly and Company. (n.d.). Clinical trial 659876. https://trials.lilly.com/en-US/trial/659876
AstraZeneca Clinical Trials. (n.d.). Study D8750C00004: Participants with obesity or overweight with at least one weight-related comorbidity. https://www.astrazenecaclinicaltrials.com/study/D8750C00004/
AbbVie News. (2025, March 3). AbbVie and Gubra announce license agreement to develop an amylin analog for the treatment of obesity. https://news.abbvie.com/2025-03-03-AbbVie-and-Gubra-Announce-License-Agreement-to-Develop-an-Amylin-Analog-for-the-Treatment-of-Obesity
Pfizer. (2025, March 12). Pfizer to acquire Metsera and its next-generation obesity portfolio. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-acquire-metsera-and-its-next-generation-obesity
BioSpace. (2025, June 15). BrightGene presents positive phase 2 data for dual GLP-1R/GIPR agonist for weight management and type 2 diabetes and preclinical data for novel amylin analog at American Diabetes Association's 85th Scientific Sessions. https://www.biospace.com/press-releases/brightgene-presents-positive-phase-2-data-for-dual-glp-1r-gipr-agonist-for-weight-management-and-type-2-diabetes-and-preclinical-data-for-novel-amylin-analog-at-american-diabetes-associations-85th-scientific-sessions
Dahl, K., et al. (2025). Amycretin, a novel, unimolecular GLP-1 and amylin receptor agonist administered subcutaneously: Results from a phase 1b/2a randomised controlled study. The Lancet, 406(10499), 149–162. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736%2825%2901185-7/abstract
Metsera. (n.d.). Pipeline. https://metsera.com/pipeline/
Gibney, M. (2025, November 6). Why Pfizer and Novo are duking it out over weight loss startup Metsera. PharmaVoice. https://www.pharmavoice.com/news/pfizer-novo-nordisk-metsera-weight-loss-deal-billions/804804/