In recent years, drug development targeting TLRs has advanced rapidly. Their diverse indications and substantial market potential have attracted increasing investment and attention.
In February 2025, Tivic Health obtained the global exclusive license for the TLR5 agonist Entolimod from Statera Biopharma, initially for the treatment of acute radiation syndrome, with potential expansion into neutropenia, immunosenescence, and vaccine adjuvant applications. Earlier in 2024, Exicure signed a patent licensing agreement with Bluejay Therapeutics, transferring the rights to develop Cavrotolimod for hepatitis. This licensing model accelerates the development of the candidate drug while allowing Exicure to retain economic interests.
Given their broad clinical potential and research value, a deeper understanding of the biological characteristics and mechanisms of TLRs is essential for advancing drug development in this area.
TLRs: History, Structure, and Function
Toll-like receptors (TLRs) are members of the pattern recognition receptor (PRR) family and play a critical role in initiating innate immune responses by detecting potentially harmful pathogens.
The discovery of TLRs traces back to the identification and functional characterization of the toll gene and its protein in Drosophila. Subsequent research revealed the structural and functional conservation of the TLR family, laying the foundation for understanding the molecular mechanisms by which TLRs mediate immune signaling in mammals.
TLRs are type I transmembrane receptors composed of an extracellular domain and an intracellular TIR domain. The extracellular domain recognizes microbial components, while the TIR domain recruits downstream signaling molecules, ultimately activating the transcription of genes involved in inflammation and antimicrobial defense.
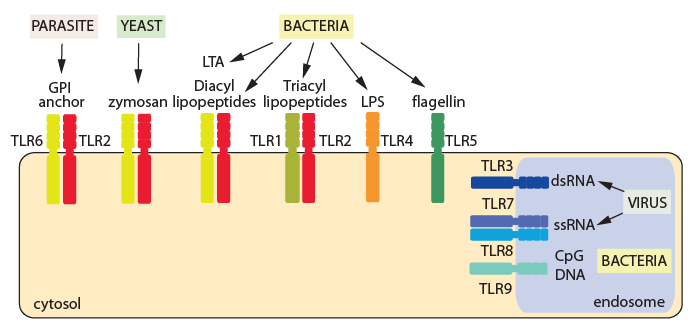
Based on subcellular localization and the pathogen-associated components they recognize, TLRs can be broadly divided into two categories. The first group is located on the cell surface and detects microbial cell wall components. For example, TLR4 recognizes lipopolysaccharide (LPS) in bacterial cell walls, while TLR2/1 and TLR2/6 recognize lipopeptides. The second group is positioned on intracellular vesicular membranes (including endosomes, the endoplasmic reticulum, and lysosomes) and recognizes nucleic acid ligands. For instance, TLR3 detects viral double-stranded RNA; TLR7 and TLR8 detect viral single-stranded RNA; and TLR9 detects CpG-containing DNA. This spatial segregation of ligand recognition ensures that the immune system senses infection with high specificity while minimizing the risk of autoimmunity.
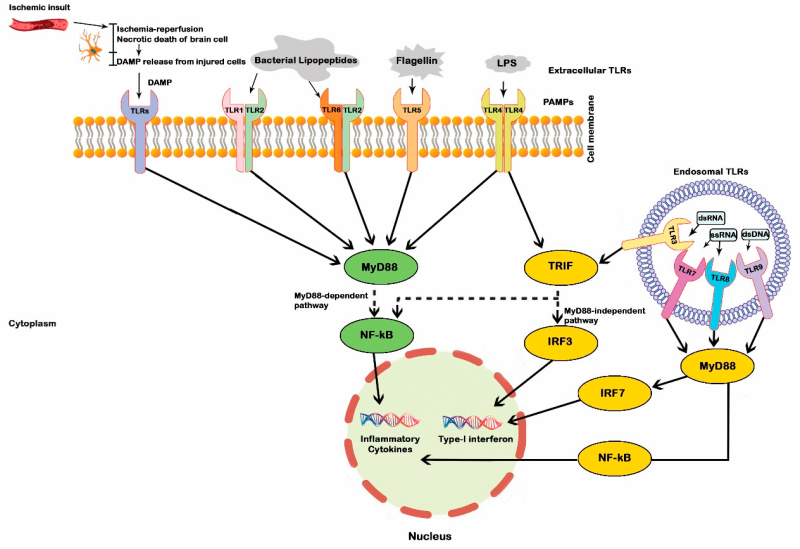
Upon activation, the TIR domain of a TLR recruits distinct adaptor proteins that determine the nature and intensity of downstream signaling. Two major adaptor proteins are involved: MYD88 (used by TLR1, 2, 4, 5, 6, 7, 8, and 9) and TRIF (used by TLR3 and TLR4). TLR4 is the only receptor capable of activating both the MyD88 pathway—which induces pro-inflammatory cytokine production—and the TRIF pathway, which leads to the production of interferons (IFNs) and interferon-stimulated genes (ISGs). As such, TLR4 is the most extensively studied member of the TLR family.
Additionally, TLR1, 2, 4, and 6 require a second adaptor, TIRAP, to recruit MYD88, whereas TLR4 requires TRAM to recruit TRIF. These adaptors assemble multiple downstream proteins, including kinases, to initiate distinct signaling cascades.
TLRs primarily activate three signaling pathways: the MAP kinase pathway (ERK, p38, and JNK), the NF-κB pathway, and the IRF pathway. These pathways ultimately promote the nuclear translocation of key transcription factors such as NF-κB and IRFs, triggering the expression of immune-response genes. Through these mechanisms, TLRs induce the production of pro-inflammatory cytokines (such as IL-6, TNF-α, and IL-12), anti-inflammatory cytokines (such as IL-10), type I interferons (IFN-α/β), and various immunoregulatory molecules, thereby modulating both innate and adaptive immune responses.
Advances in Drug Development Targeting TLRs
Activation of TLR signaling pathways induces immune responses; therefore, TLR agonists can be used as immunotherapies or vaccine adjuvants for the treatment of cancer, allergies, and infectious diseases. In contrast, TLR antagonists modulate excessive immune activation and hold therapeutic potential for chronic inflammatory and autoimmune disorders.
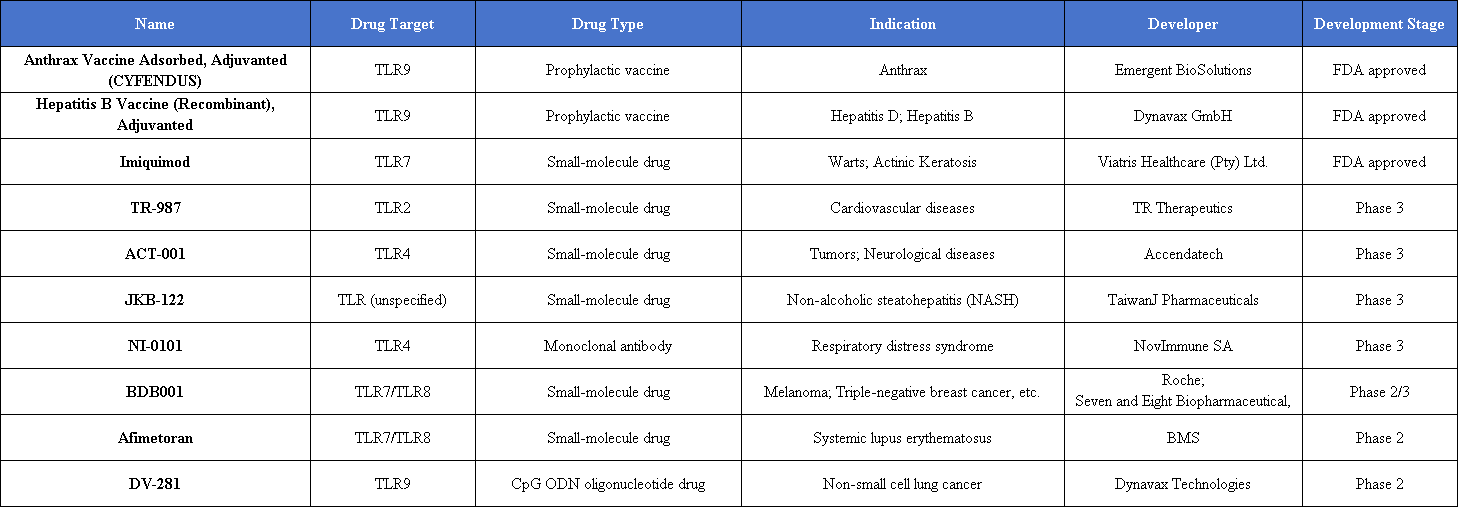
At present, most approved and clinically advanced therapeutics targeting TLRs focus on TLR agonism. Several TLR-targeting agents have already received regulatory approval, including Anthrax Vaccine Adsorbed developed by Emergent BioSolutions, the adjuvanted hepatitis B vaccine HEPLISAV-B developed by Dynavax Technologies, and Imiquimod from Viatris Healthcare.
Other TLR-targeting drug candidates currently in clinical development include:
Reference
Accendatech. (n.d.). KYSL product page. https://www.accendatech.com/en/kysl.aspx?CID=69
Ashayeri Ahmadabad, R., Mirzaasgari, Z., Gorji, A., & Khaleghi Ghadiri, M. (2021). Toll-like receptor signaling pathways: Novel therapeutic targets for cerebrovascular disorders. International Journal of Molecular Sciences, 22(11), 6153. https://doi.org/10.3390/ijms22116153
BioSpace. (n.d.). Seven and Eight Biopharmaceuticals Inc. announces clinical collaboration. https://www.biospace.com/seven-and-eight-biopharmaceuticals-inc-announces-clinical-collaboration
British Society for Immunology. (n.d.). Pattern recognition receptors (PRRs): Toll-like receptors. https://www.immunology.org/public-information/bitesized-immunology/receptors-molecules/pattern-recognition-receptors-prrs-toll
ClinicalTrials.gov. (n.d.). Study record: NCT00761371. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT00761371
ClinicalTrials.gov. (n.d.). Study record: NCT01808469. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT01808469
ClinicalTrials.gov. (n.d.). Study record: NCT03326752. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT03326752
ClinicalTrials.gov. (n.d.). Study record: NCT04255069. U.S. National Library of Medicine. https://clinicaltrials.gov/study/NCT04255069
ClinicalTrials.gov. (n.d.). Study record: NCT04895696. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT04895696
ClinicalTrials.gov. (n.d.). Study record: NCT06707090. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT06707090
Exicure Inc., & Bluejay Therapeutics Inc. (2024). Exicure Inc. and Bluejay Therapeutics Inc. enter into a patent license agreement to develop cavrotolimod for the treatment of hepatitis. https://investors.exicuretx.com/news/news-details/2024/Exicure-Inc.-and-Bluejay-Therapeutics-Inc.-Enter-into-a-Patent-License-Agreement-to-Develop-Cavrotolimod-for-the-Treatment-of-Hepatitis/default.aspx
Sameer, A. S., & Nissar, S. (2021). Toll-like receptors (TLRs): Structure, functions, signaling, and role of their polymorphisms in colorectal cancer susceptibility. Biomed Research International, 2021, 1157023. https://doi.org/10.1155/2021/1157023
Tivic Health. (n.d.). Tivic Health extends its worldwide license of TLR5 agonist entolimod to include the treatment of neutropenia. https://tivichealth.com/tivic-health-extends-its-worldwide-license-of-tlr5-agonist-entolimod-to-include-the-treatment-of-neutropenia/
U.S. Food and Drug Administration. (n.d.). CYFENDUS. https://www.fda.gov/vaccines-blood-biologics/vaccines/cyfendus
U.S. Food and Drug Administration. (n.d.). HEPLISAV-B. https://www.fda.gov/vaccines-blood-biologics/vaccines/heplisav-b