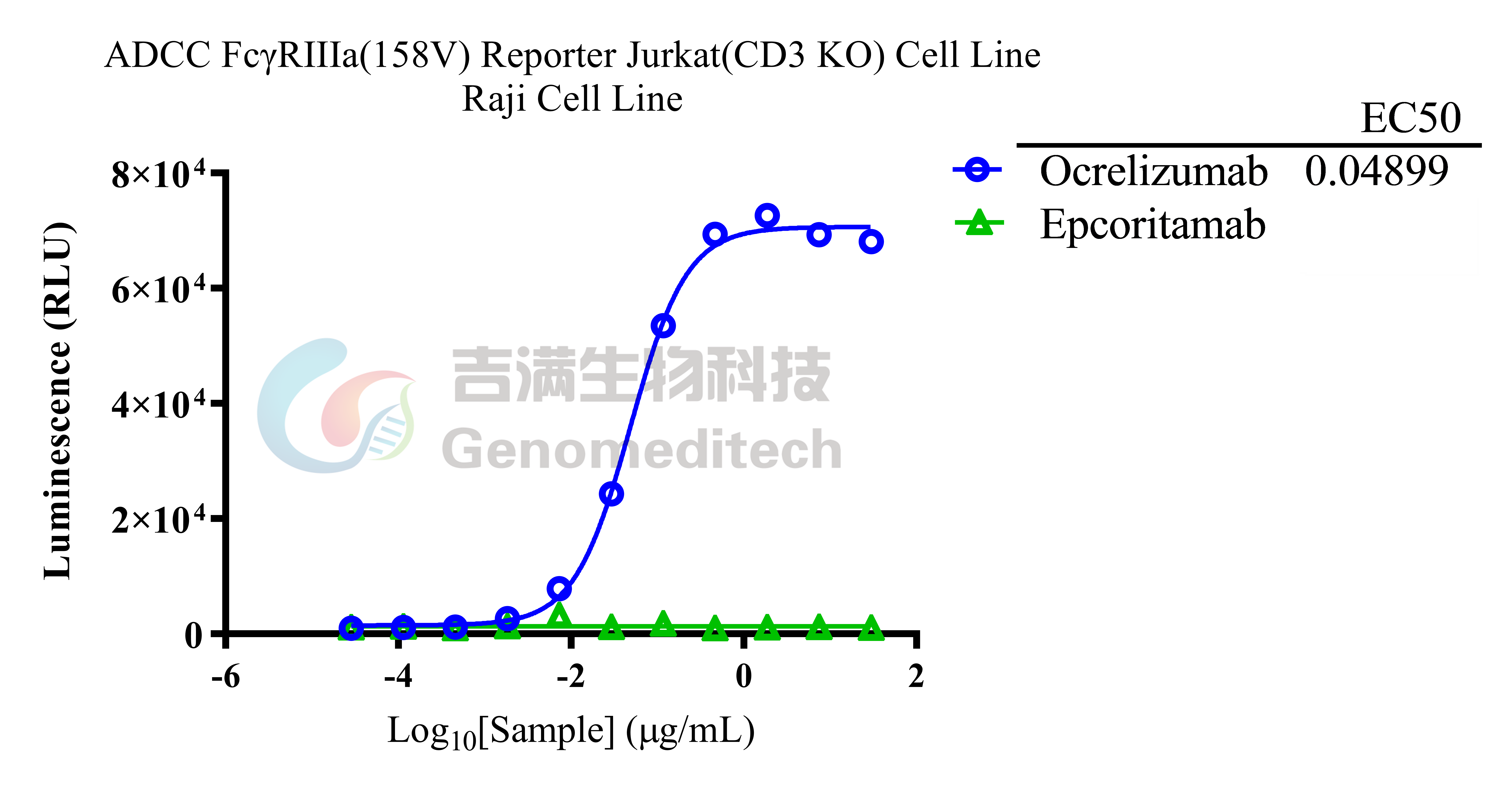
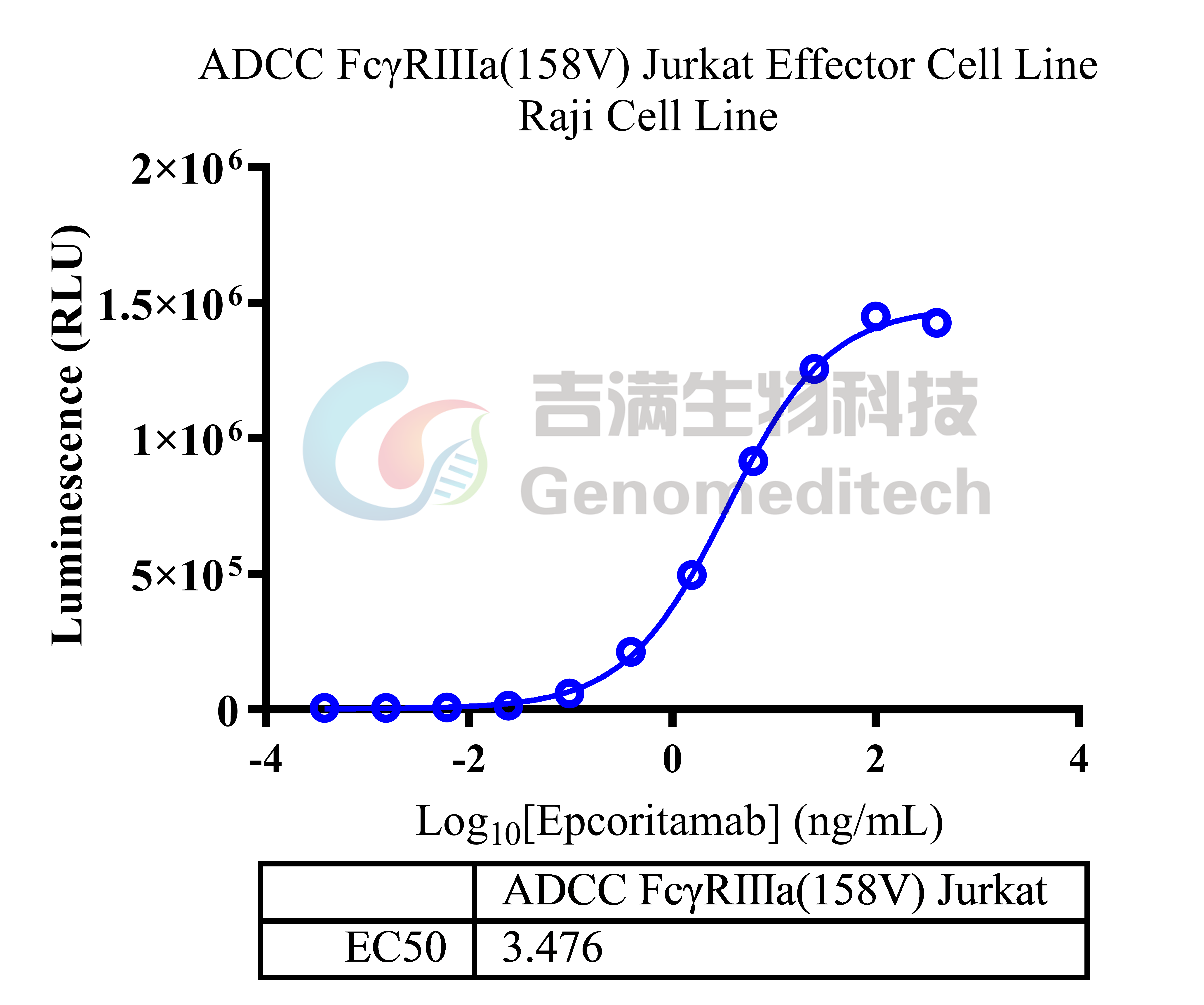
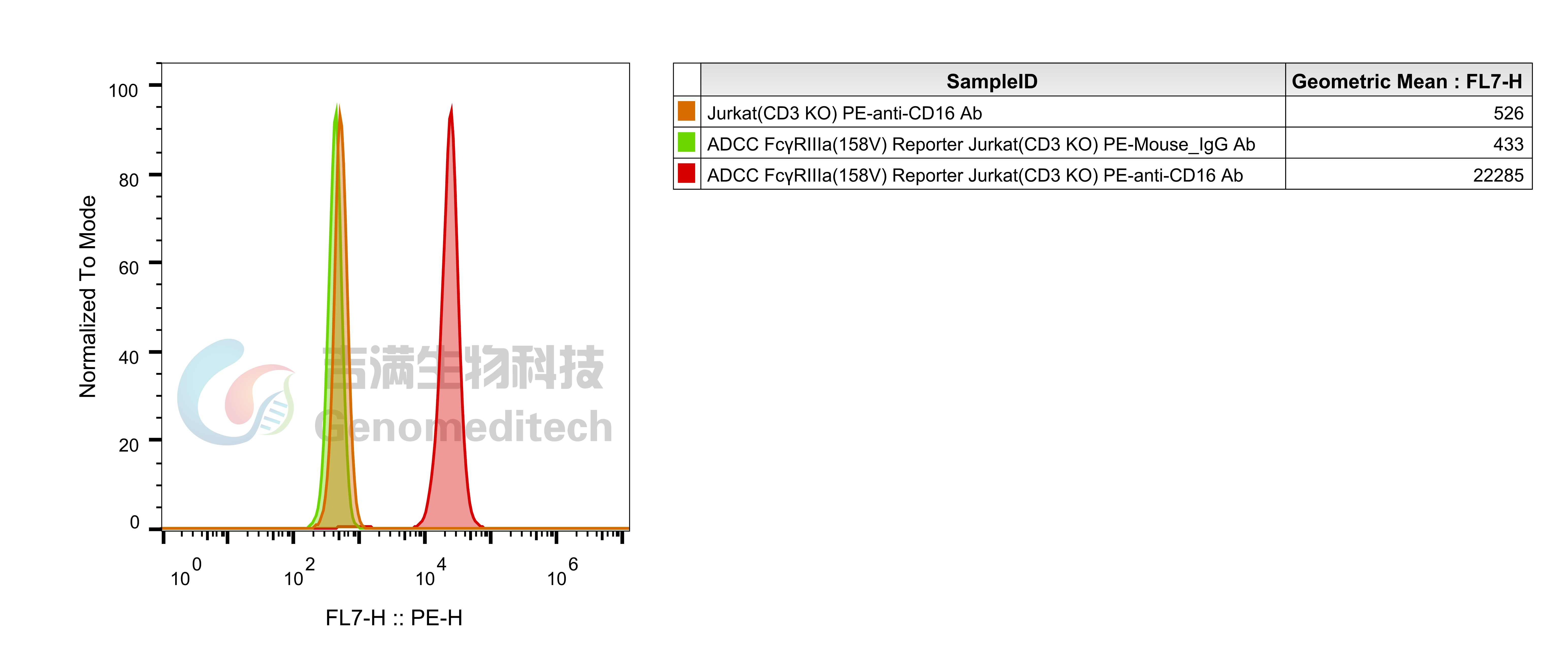
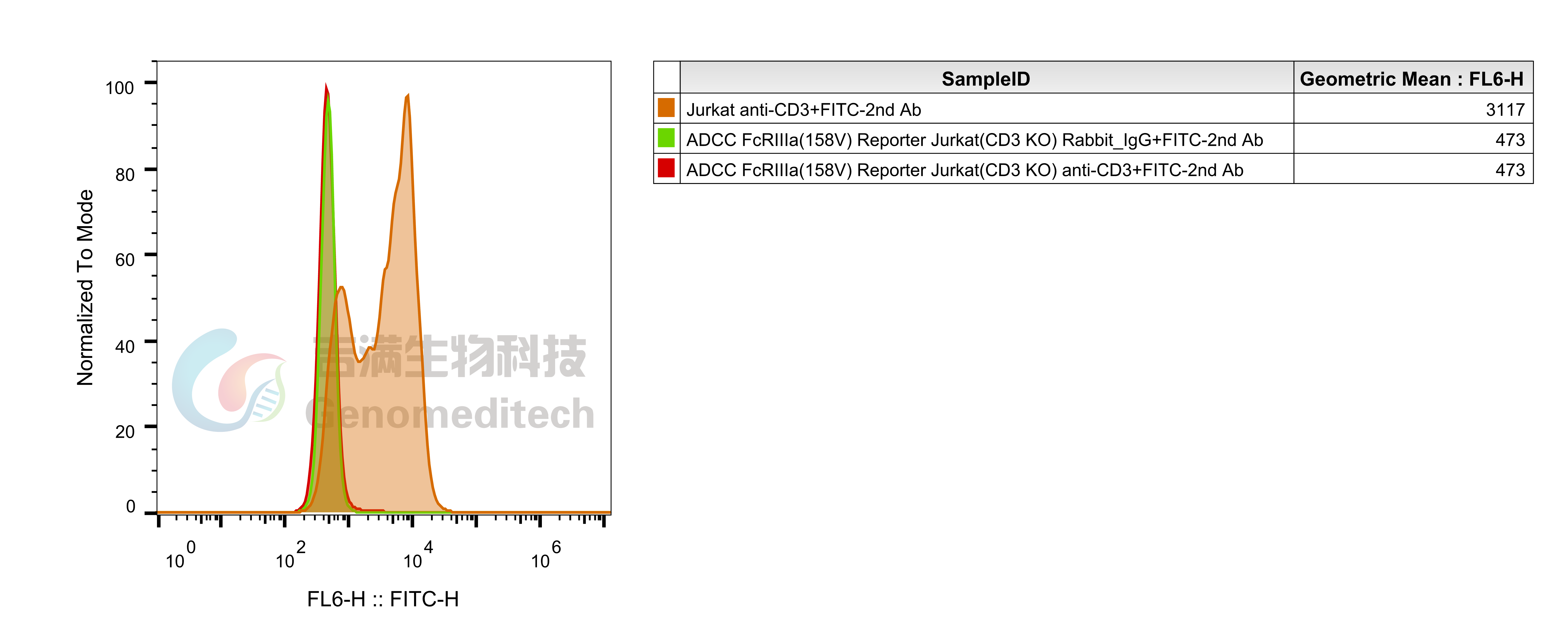
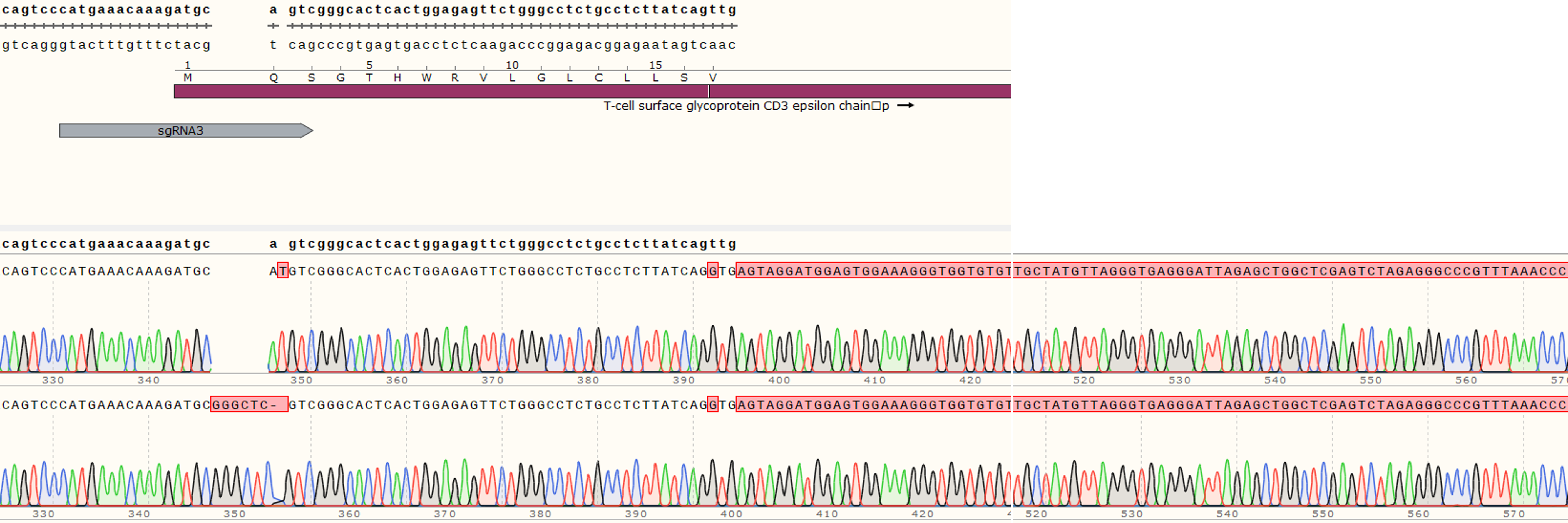
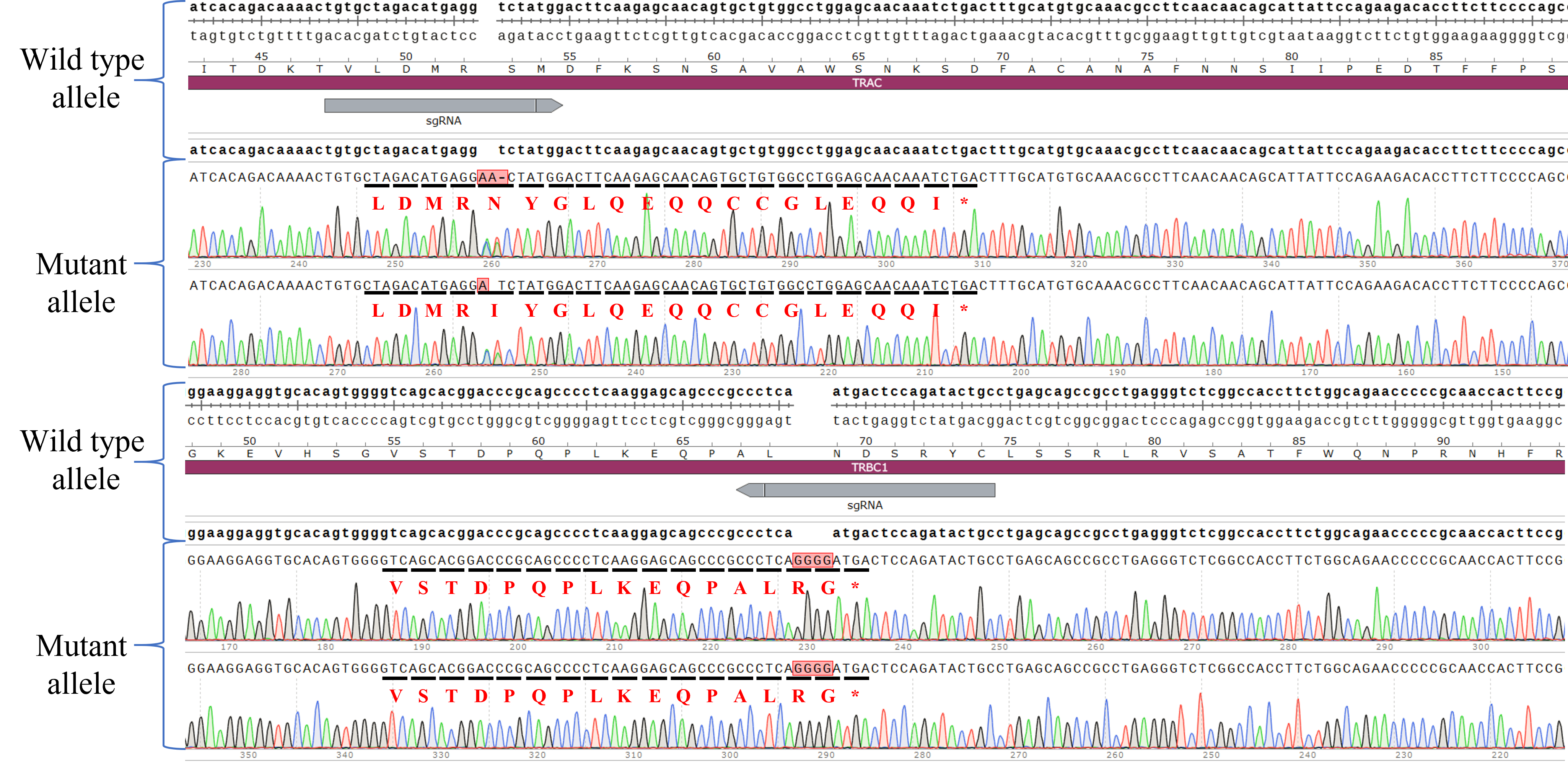
| Cat. No: GM-C39293 Product: ADCC FcγRIIIa(158V) Reporter Jurkat(CD3 KO) Cell Line ADCC, or antibody-dependent cell-mediated cytotoxicity, refers to the process by which immune cells expressing Fc receptors directly kill target cells that specifically bind to antibodies through recognition of the Fc region of the antibodies. Nowadays, the mechanism of ADCC is used to detect and evaluate the efficacy of antibodies or target cells. Antibodies bind to target antigens on the cell surface. If the Fc region of the antibody simultaneously binds to the FcγRIIIa receptor on the surface of effector cells (primarily natural killer cells), the two types of cells undergo multiple cross-linking, leading to the activation of the ADCC signaling pathway. The 158V variant is a polymorphism where valine (V) replaces phenylalanine (F) at position 158, and the 158V mutation exhibits high affinity. ADCC FcγRIIIa(158V) Reporter Jurkat(CD3 KO) Cell Line is a clonal stable Jurkat cell line that knockout Endogenous CD3 complex and constitutive expression of human FcγRIIIa(158V) gene, along with signal-dependent expression of a luciferase reporter gene. This design enables cells to specifically respond to receptor-mediated ADCC activation signals. By measuring the bioluminescence intensity of luciferase within the cells, the antibody activity of the ADCC pathway can be accurately quantified. This system features strong signals and low background, making it particularly suitable for functional activity and mechanism validation studies of bispecific T cell engager (TCE) drugs. | 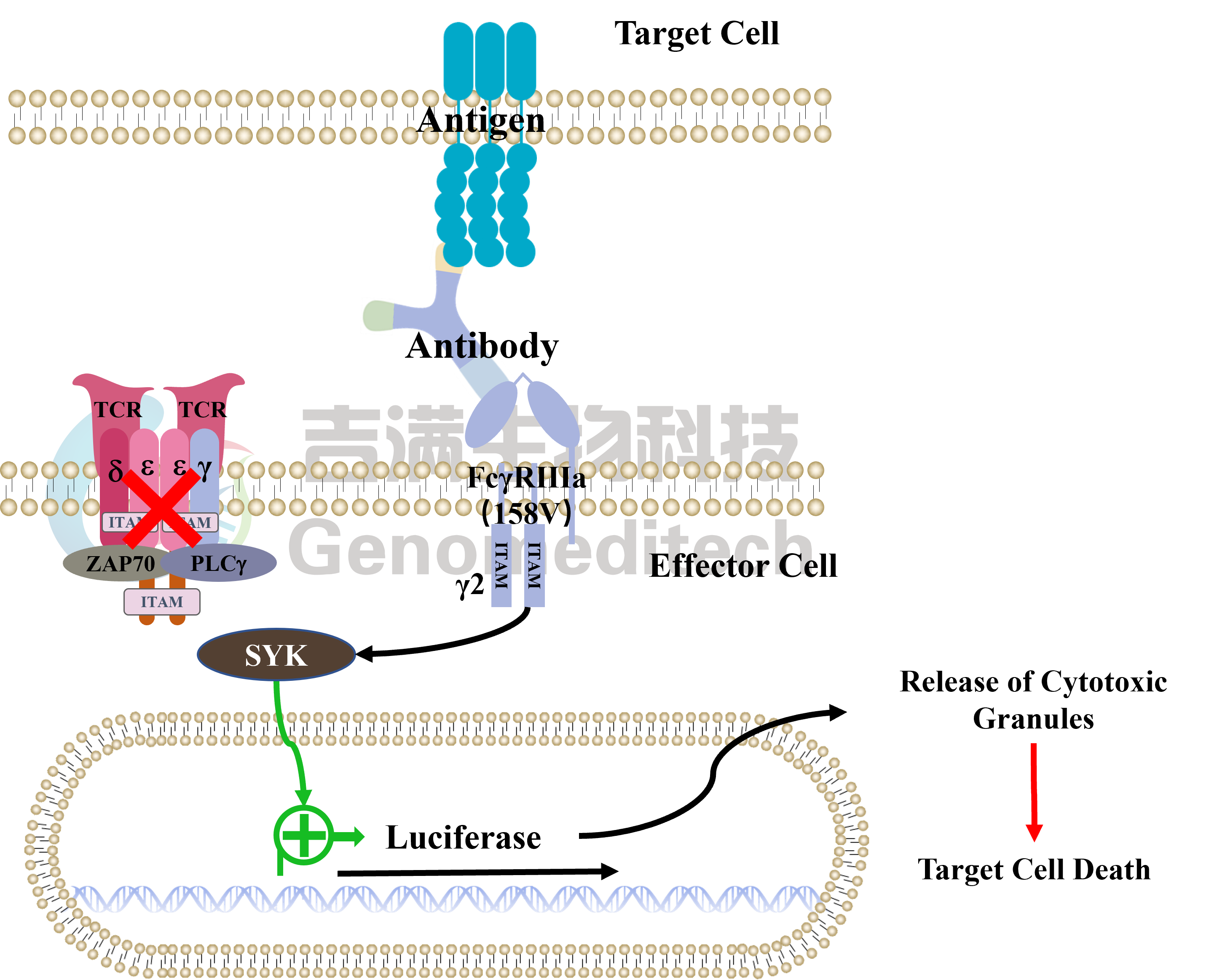 |