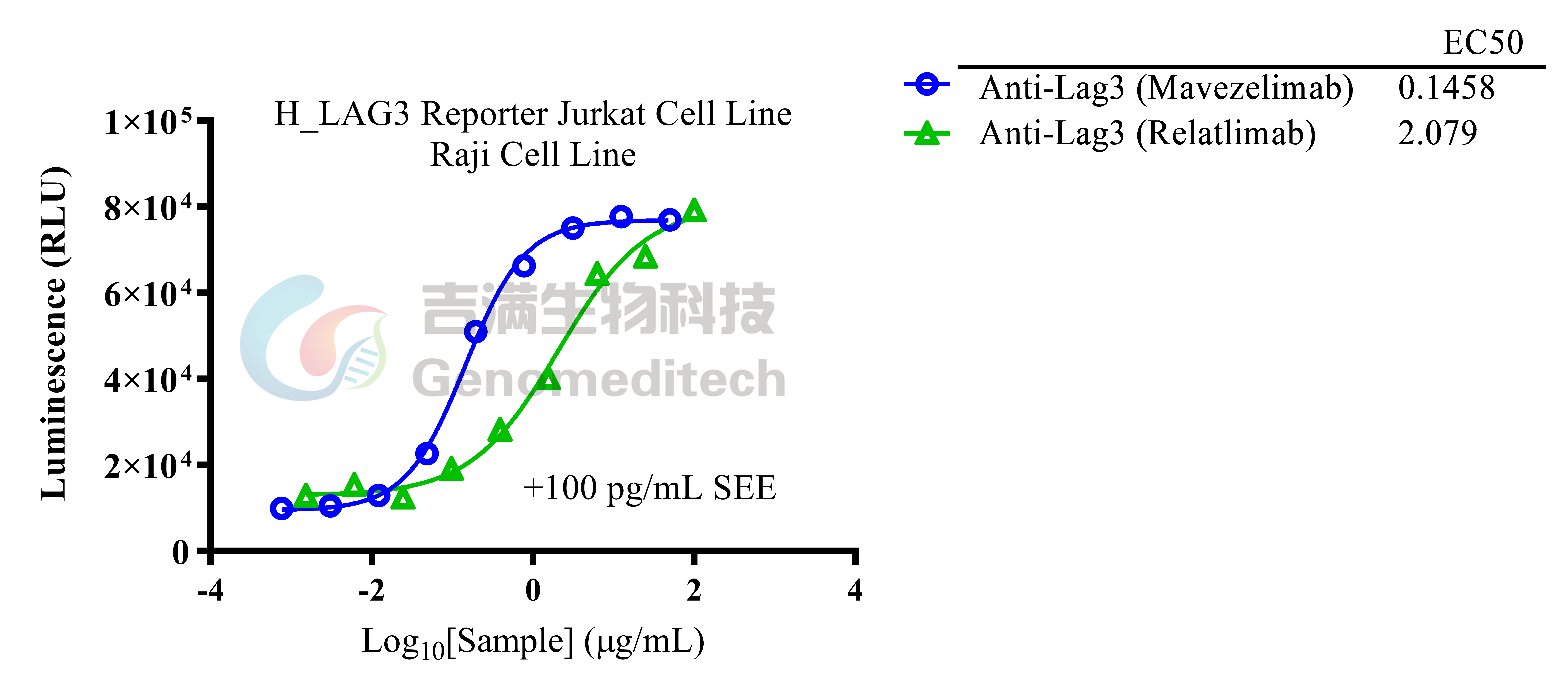
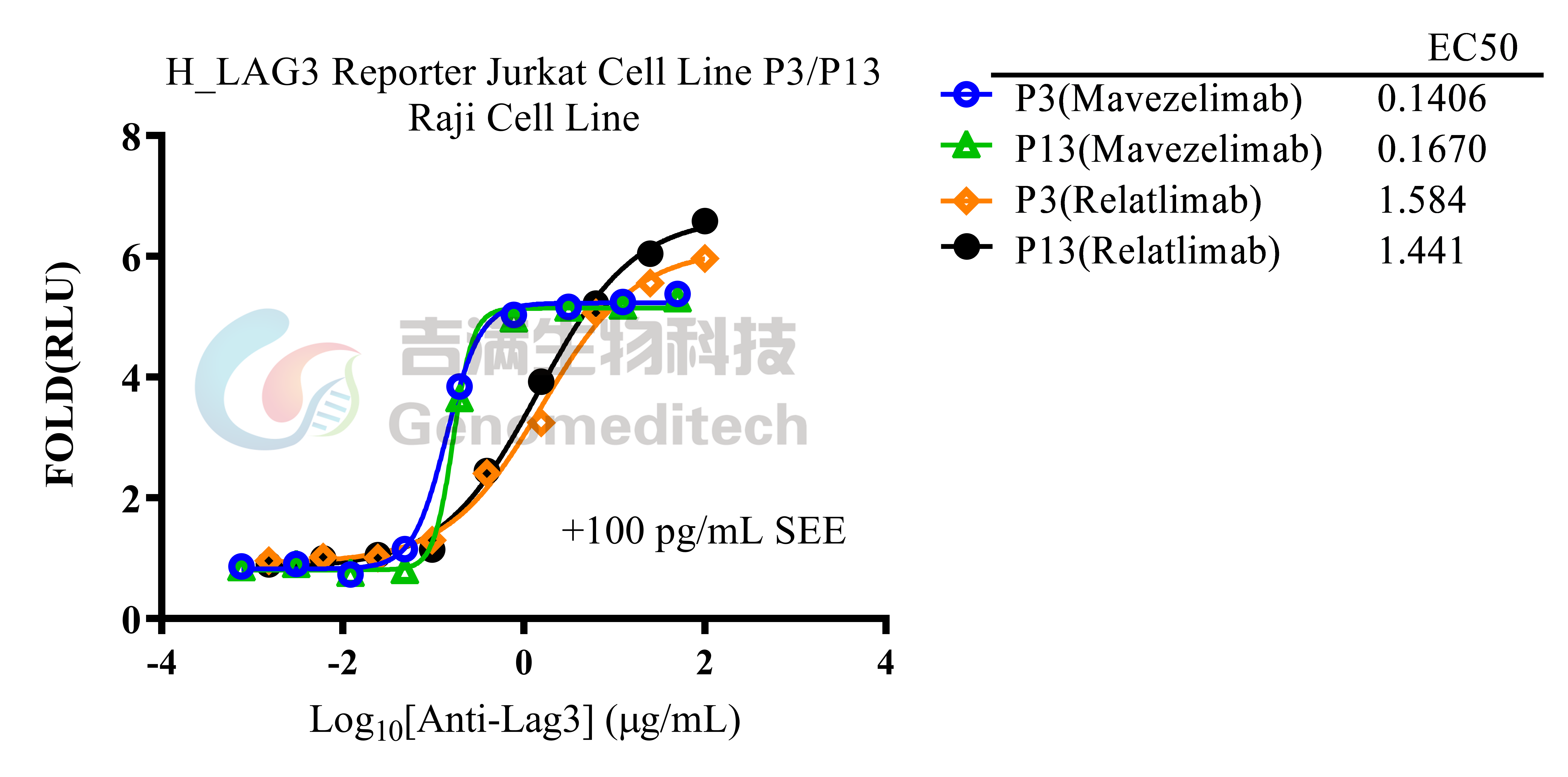
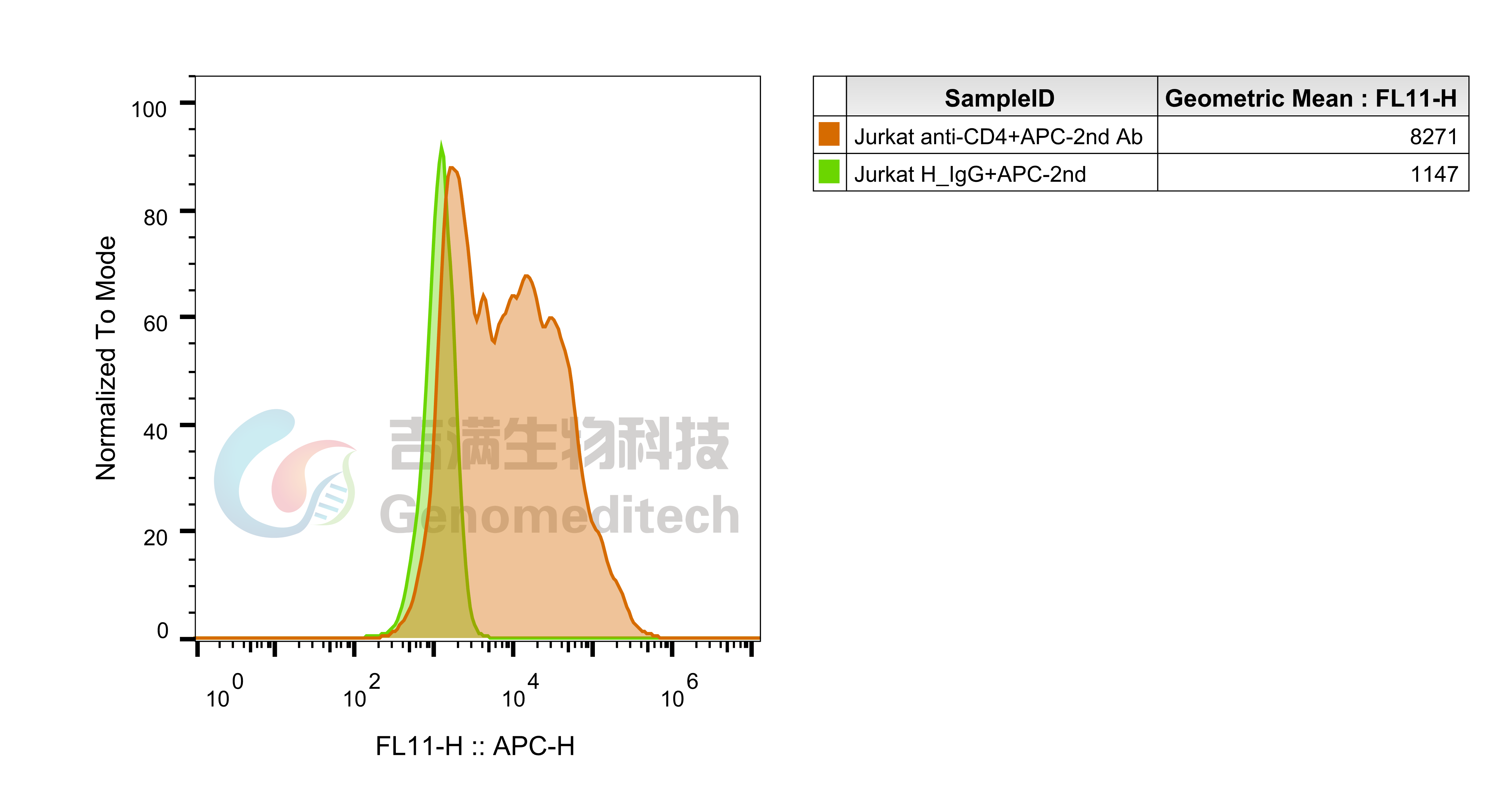
| Cat. No: GM-C20096 Product: H_LAG3 Reporter Jurkat Cell Line LAG3 is an immune checkpoint receptor expressed on activated T cells, Tregs, NK cells, and some DCs. Similar to CD4, it binds MHC class II with higher affinity, negatively regulating T cell activation and maintaining immune homeostasis. LAG3 limits immune responses to self-antigens, infections, and tumors, making it a key target in cancer and autoimmune immunotherapy. LAG3 suppresses T cell activation and proliferation by binding to ligands like MHC class II and interacting with signaling adapters to inhibit TCR signaling. This reduces calcium flux, activation marker expression, and cytokine production. In Tregs, LAG3 enhances immunosuppressive functions and can modulate DC activity, contributing to an immunosuppressive tumor microenvironment. H_LAG3 Reporter Jurkat Cell Line is a clonal stable Jurkat cell line constructed using lentiviral technology, inducible expression of the human LAG3 gene, endogenously expression of the TCR-CD3 complex and CD4 gene, along with signal-dependent expression of a luciferase reporter gene. Switch-On Reagent is required for induction before use. When LAG3 binds to MHCII, it activates downstream signaling pathways, inhibit the expression of luciferase. Blockade antibodies can block this inhibitory signal transmission, restore the activation of T cells. The luciferase activity measurement indicates the activation level of the signaling pathway and can thus be used to evaluate the in vitro effects of drugs related to LAG3. | 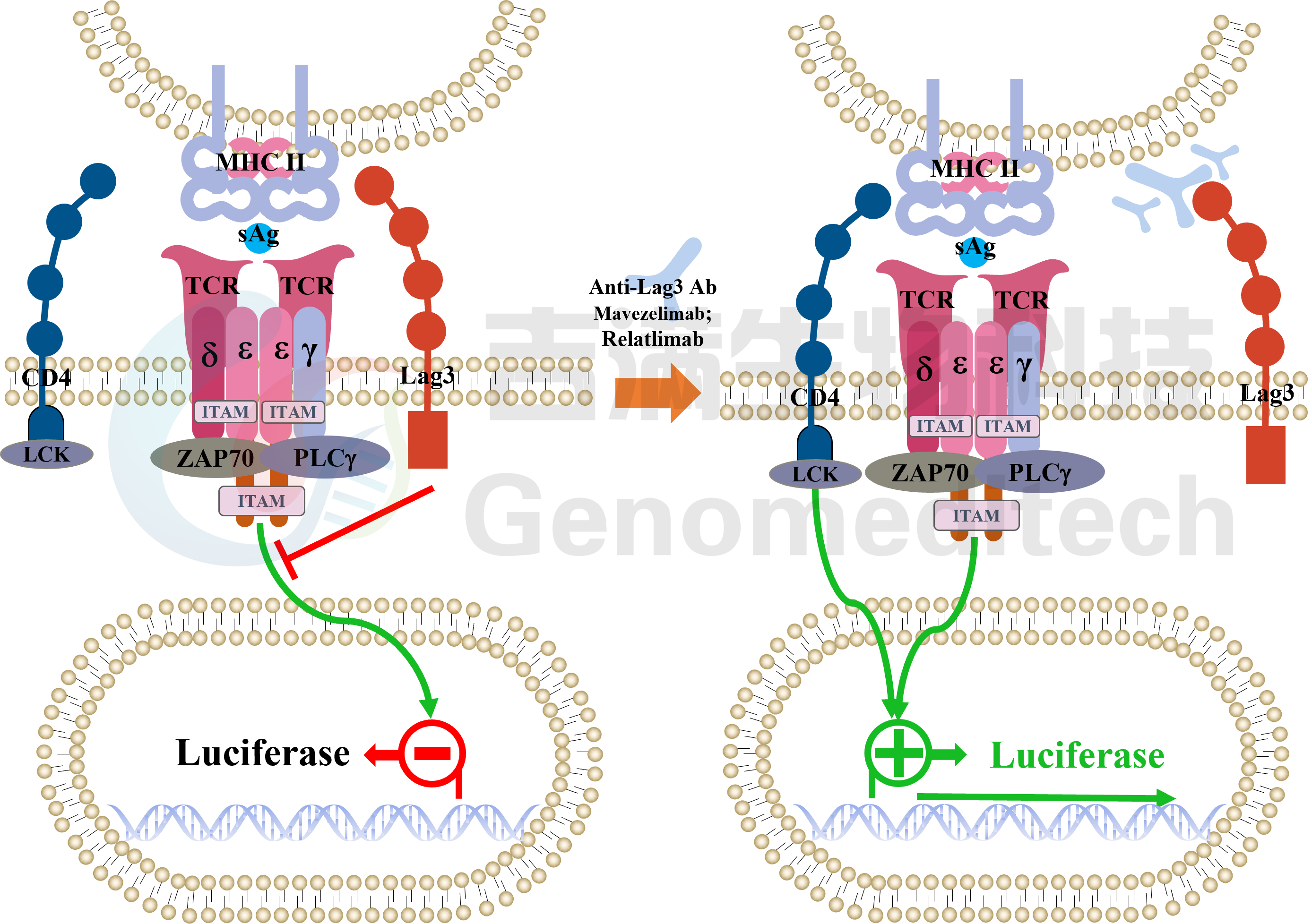 |