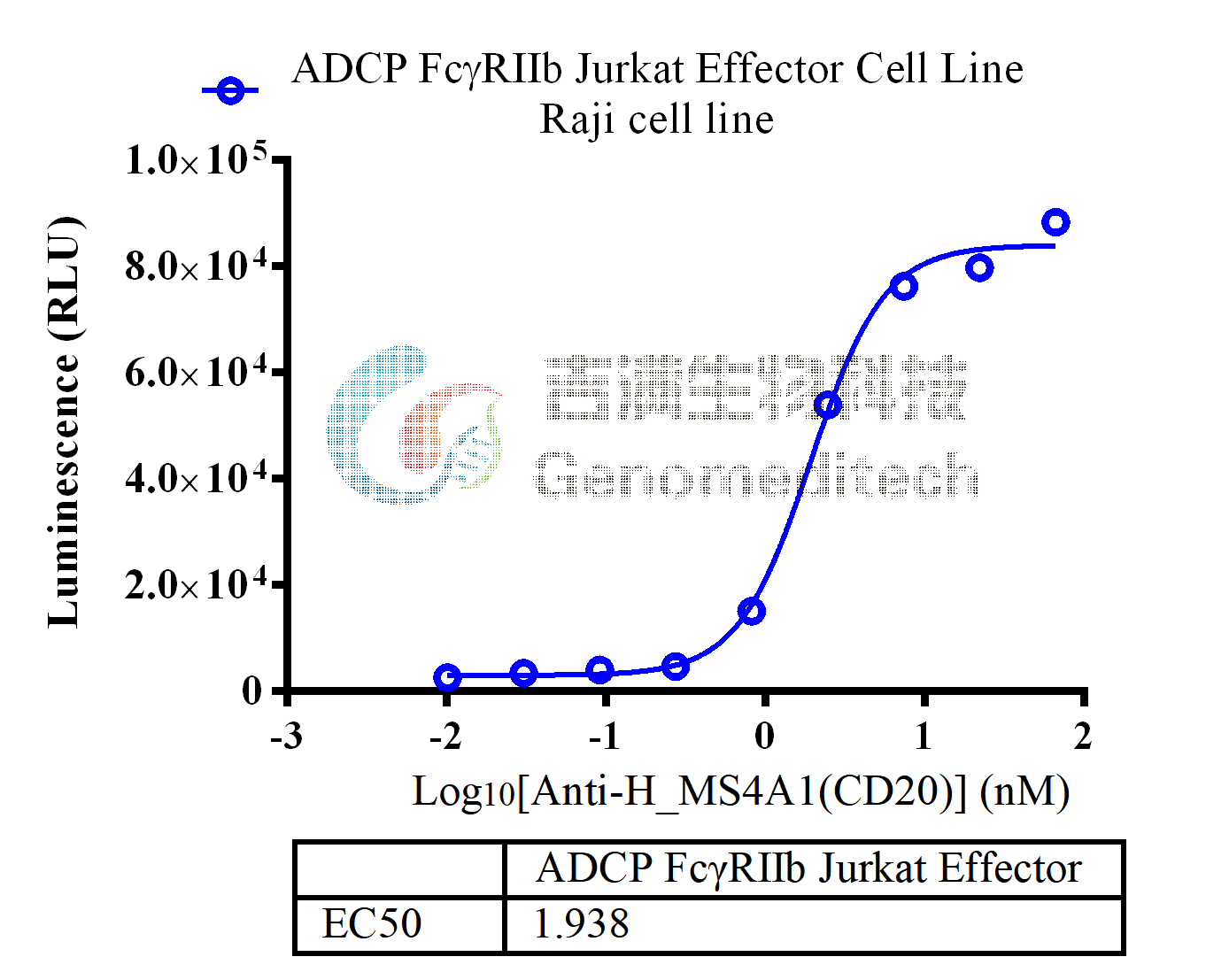
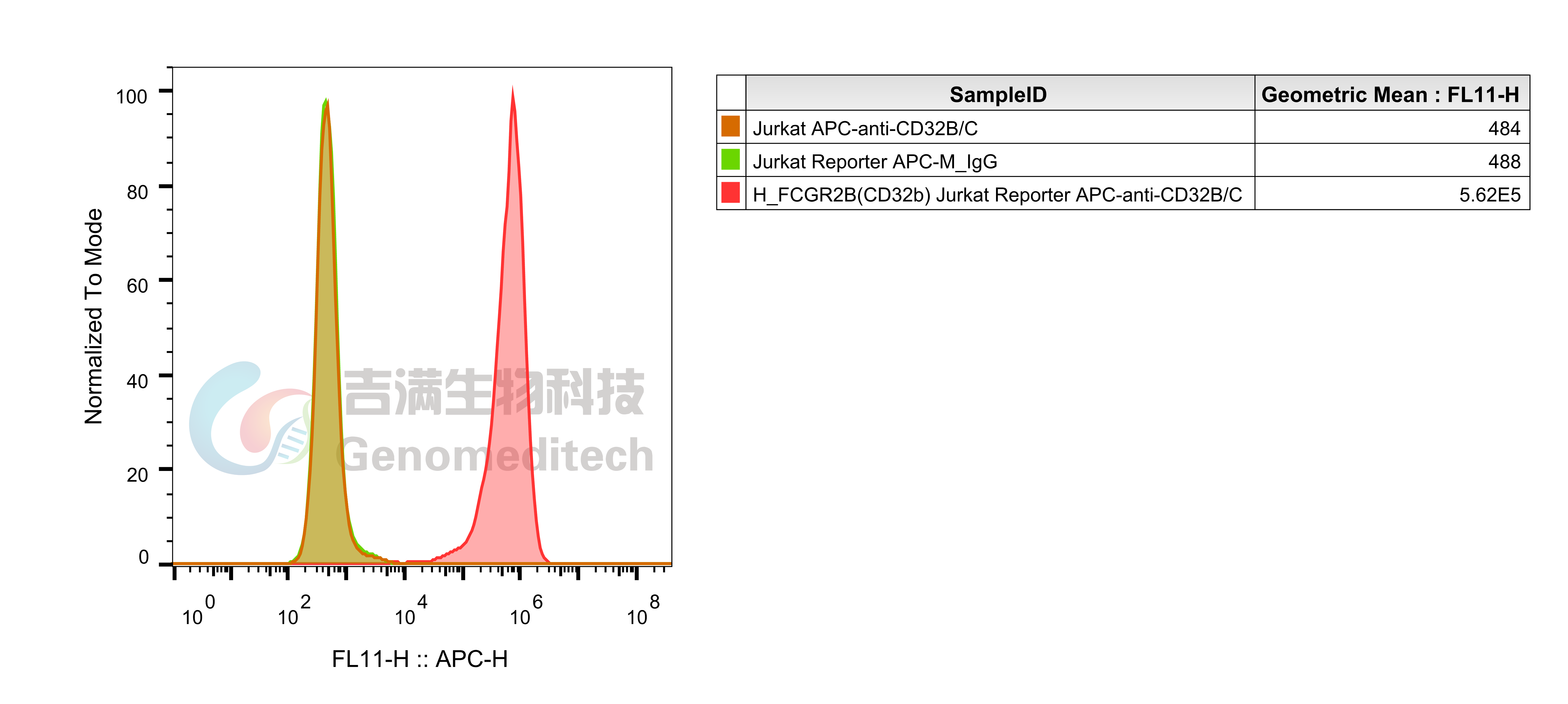
| Cat. No: GM-C26215 Product: ADCP FcγRIIb Jurkat Effector Cell Line ADCP, or antibody-dependent cellular phagocytosis, is a process where immune cells with Fc receptors recognize the Fc region of antibodies and phagocytose antibody-bound target cells. This mechanism is now used to evaluate antibody efficacy. The antibody's Fab region binds to the target antigen, while its Fc region interacts with FcγRIIb receptors on effector cells (mainly macrophages), triggering ADCP and leading to target cell phagocytosis. Traditional ADCP assays use donor-derived macrophages, but these cells are variable, hard to prepare, and prone to high background signals. FcγRIIb (Fc gamma receptor IIb) is a low-affinity receptor for the Fc region of immunoglobulin G (IgG) and is the only Fcγ receptor with inhibitory functions. It is expressed on various immune cells, including B cells, dendritic cells, macrophages, and neutrophils. FcγRIIb plays a critical role in regulating immune responses by dampening activation signals, maintaining immune homeostasis, and preventing excessive inflammation or autoimmunity. ADCP FcγRIIb Jurkat Effector Cell Line is a clonal stable Jurkat cell line constructed using lentiviral technology, constitutive expression of the FcγRIIb gene, along with signal-dependent expression of a luciferase reporter gene. When IgG binds to target cells and effector cells, it leads to the expression of luciferase, which can be used to evaluate the biological activity of antibodies in the mechanism of ADCP. | 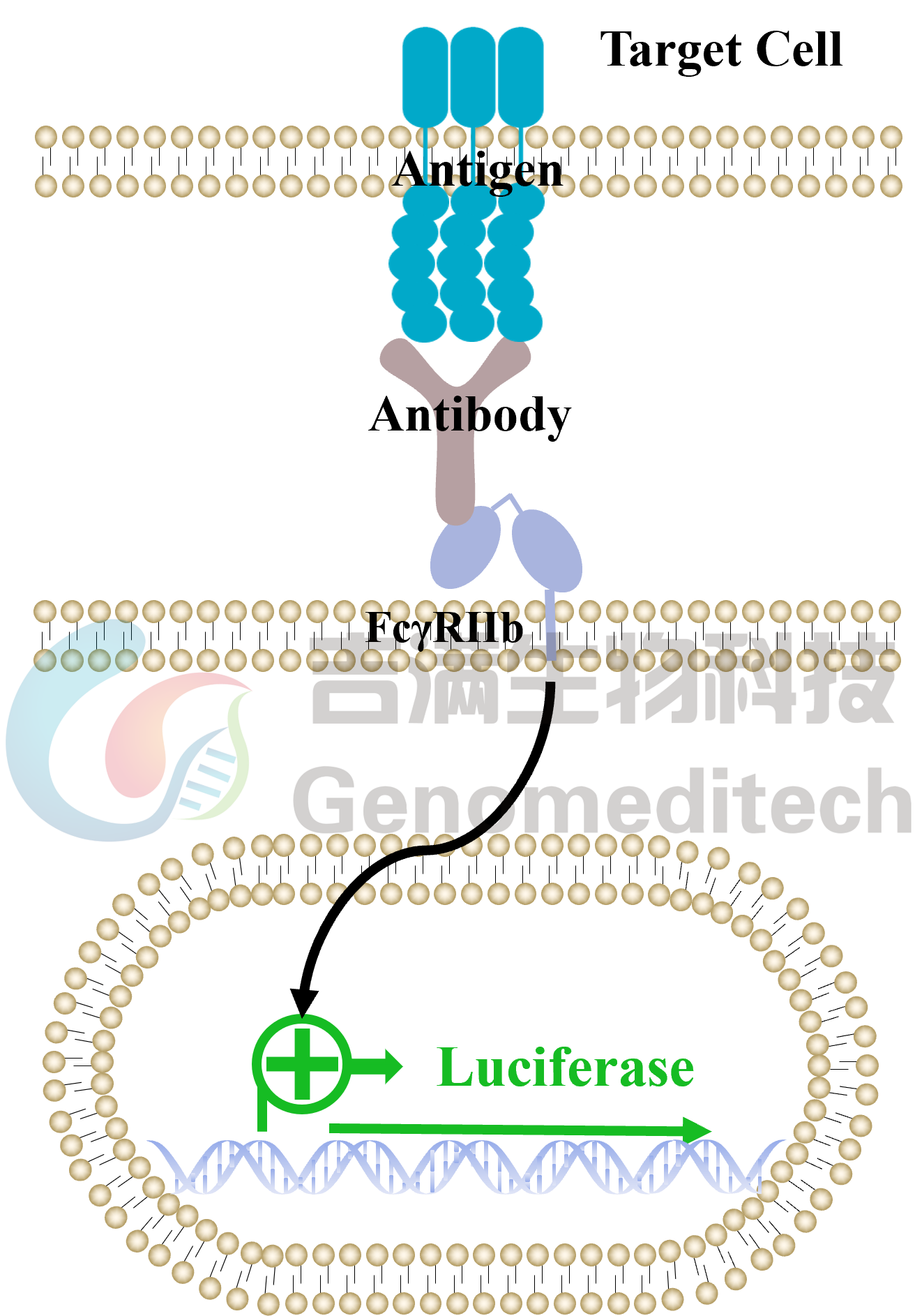 |