The PD-1 target is a milestone in the field of cancer immunotherapy. PD-1/PD-L1–based therapies have become a cornerstone of cancer treatment, supporting a multi-billion-dollar drug market and driving the development of second-generation immunotherapies, such as PD‑1/VEGF bispecific antibodies and PD‑1–combined ADCs, effectively addressing previously unmet clinical needs and leading recent advances in oncology drug development.
While PD-1 inhibitors continue to play a central role in oncology, a class of “opposite mechanism” drugs—PD-1 agonists—is emerging in the field of autoimmune diseases. From rheumatoid arthritis (RA) to systemic lupus erythematosus (SLE) and primary Sjögren’s syndrome (pSS), this approach may provide new therapeutic options for autoimmune patients.
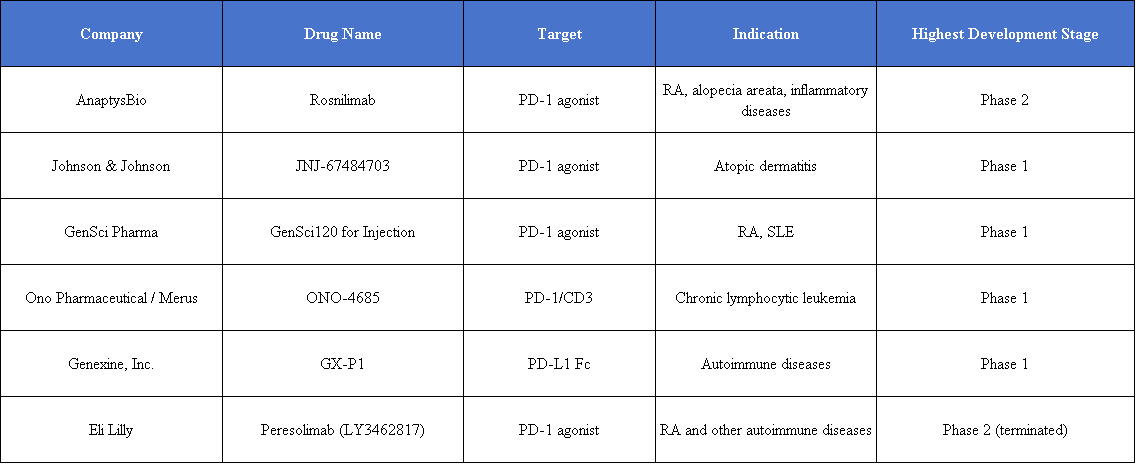
Among the PD-1 agonists in development, AnaptysBio’s Rosnilimab has taken the lead by completing key Phase 2b clinical validation in RA, becoming one of the most representative pioneers of this mechanism in autoimmune diseases.
Meanwhile, global autoimmune leaders such as Sanofi have begun to take action. In December 2025, Sanofi reached an equity investment and strategic collaboration with biotechnology company InduPro to jointly develop bispecific PD-1 agonists through preclinical studies and IND application research, strengthening efforts in this emerging field.
The market potential is also significant. According to a report by Future Market Insights, the global autoimmune drug market was valued at USD 168.6 billion in 2025 and is expected to reach USD 226.2 billion by 2035, with a compound annual growth rate of 3.0%. Compared with the highly competitive oncology field, autoimmune diseases represent a promising new area for PD-1 agonists.
Reversing Immune Activation for Autoimmune Therapy
The core concept of PD-1 agonists represents a reverse approach to immune modulation. In cancer, PD-1 inhibitors block the PD-1/PD-L1 pathway, activating the immune system and enabling T cells to attack tumor cells. In autoimmune diseases, overactivated T cells are the main drivers of tissue damage. PD-1 agonists act in the opposite manner, enhancing immune inhibition, suppressing the proliferation and effector function of pathogenic T cells, and reducing pro-inflammatory cytokine release, thereby alleviating tissue damage at its source.
This bidirectional regulation is based on the central role of the PD-1/PD-L1 pathway in immune homeostasis: it is both a key node for tumor immune evasion and a checkpoint for maintaining immune tolerance. Studies have shown that in RA patients, PD-1–positive T cells can account for over 70% of T cells in inflamed joint tissue, providing a clear rationale for targeted therapy. T cell–driven autoimmune diseases such as ulcerative colitis are also becoming clinical priorities due to similar mechanisms.
The broader potential of PD-1 agonists extends to diseases including SLE, primary Sjögren’s syndrome, multiple sclerosis, and type 1 diabetes, all of which are mediated by T cells. Historically, PD-1 agonist development progressed slowly due to technical and mechanistic limitations. However, recent advances in antibody screening, AI-assisted drug design, and deeper understanding of immune mechanisms have overcome these barriers, enabling the transition from theoretical research to clinical validation.
Latest Progress: Early Signs of Promise
The development of PD-1 agonists has experienced several setbacks.
Peresolimab, previously developed by Eli Lilly, showed significant improvement in DAS28-CRP in Phase 2 RA trials, but ACR50/70 response rates were not significantly better than placebo. Its development was discontinued in the third quarter of 2024.
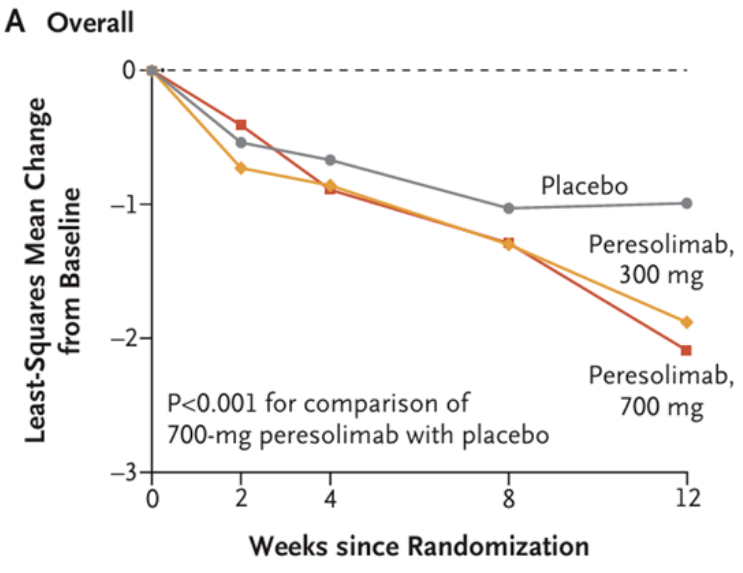
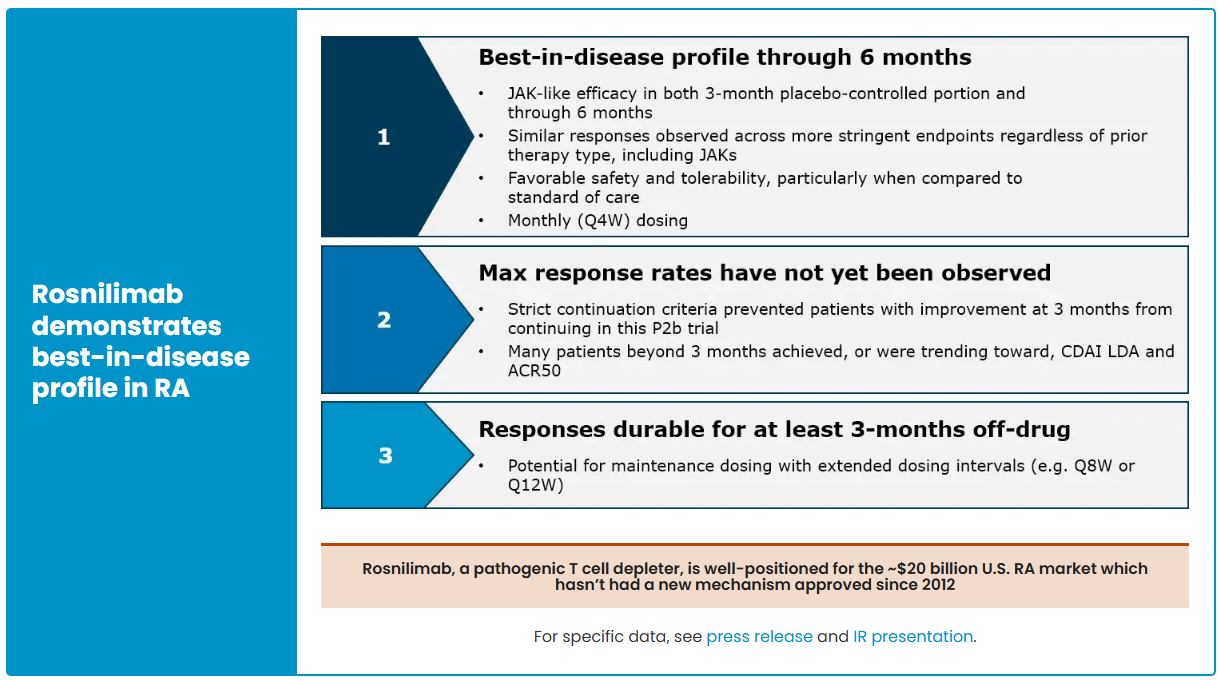
In contrast, AnaptysBio’s PD-1 agonist antibody Rosnilimab has shown strong performance. As the fastest-advancing PD-1 agonist project globally after Peresolimab, AnaptysBio reports that Rosnilimab demonstrates comprehensive advantages in both mechanism of action and clinical outcomes. The drug specifically binds to a membrane-proximal epitope of PD-1, depleting over 90% of pathogenic T cells in peripheral blood, markedly outperforming contemporaneous competitors.
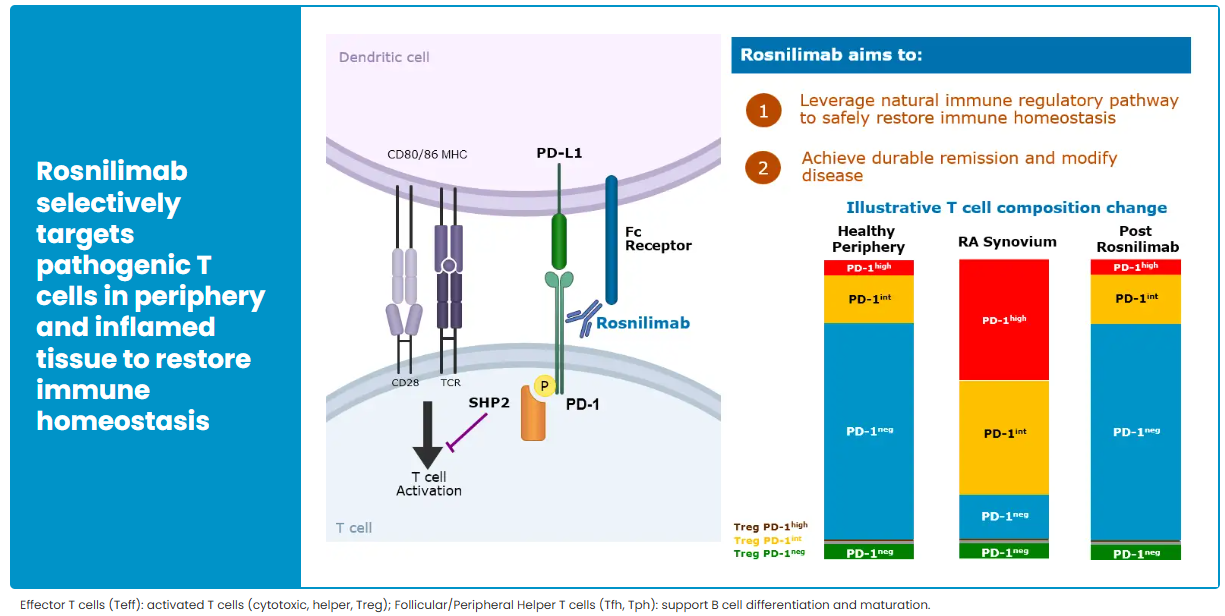
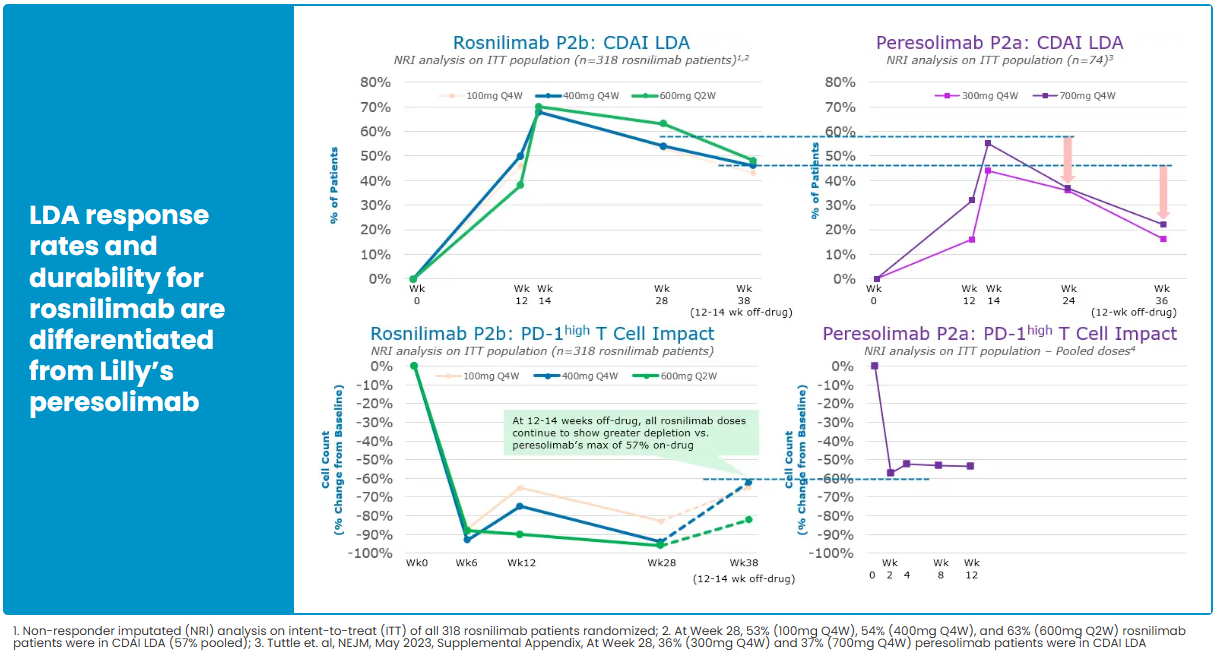
In the RA indication, Rosnilimab has completed key Phase 2b clinical validation. In February 2025, six-month CDAI and LDA (low disease activity) data were disclosed, showing response rates at a historical high for this mechanism, with an overall favorable safety profile. In June 2025, AnaptysBio further updated the complete six-month Phase 2b follow-up results, reinforcing Rosnilimab’s comprehensive advantages in depth and durability of efficacy, as well as safety, and highlighting its competitive position as a leading PD-1–exhaustion agonist in the RA market.
In terms of specific efficacy metrics, Rosnilimab demonstrated superior or comparable outcomes to approved JAK inhibitors and CTLA-4 fusion proteins in RA patients at key time points such as Weeks 14 and 28, across CDAI, ACR50, and ACR70 endpoints, further confirming its clinical potential.
However, in the UC indication, Rosnilimab has not achieved success. On November 10, 2025, the company announced that Rosnilimab failed to meet the primary and secondary endpoints in its Phase 2 clinical trial for UC, and subsequent development in this indication will be discontinued.
Currently, Rosnilimab’s development is focused on the RA indication, with anticipation for further results from the upcoming pivotal Phase 3 trials.
Looking Ahead: New Life for an Established Target
PD-1 agonists are transitioning from oncology to autoimmune diseases, demonstrating the potential of an established target to be applied in multiple contexts and explored in innovative ways to create new clinical opportunities. As more companies advance development, PD-1 agonists may enter a new wave of growth in 2026.
Reference
AnaptysBio, Inc. (2023). A study of rosnilimab in participants with moderate to severe rheumatoid arthritis (ClinicalTrials.gov Identifier NCT06041269). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT06041269
Janssen Research & Development, LLC. (2021). A study of JNJ 67484703 in participants with atopic dermatitis (ClinicalTrials.gov Identifier NCT04985812). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04985812
Changchun GeneScience Pharmaceutical Co., Ltd. (2023). Assessment of safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple subcutaneous injections of GenSci120 in healthy adult participants in China (ClinicalTrials.gov Identifier NCT07040930). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT07040930
Ono Pharmaceutical Co., Ltd. (2022). Study of ONO 4685 in patients with relapsed or refractory T cell lymphoma (ClinicalTrials.gov Identifier NCT05079282). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT05079282
Genexine, Inc. (2020). Safety and tolerability of GX P1 in healthy male volunteers (ClinicalTrials.gov Identifier NCT04298749). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04298749
Eli Lilly and Company. (2019). A safety study of LY3462817 and LY3509754 in participants with psoriasis (ClinicalTrials.gov Identifier NCT04152382). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04152382