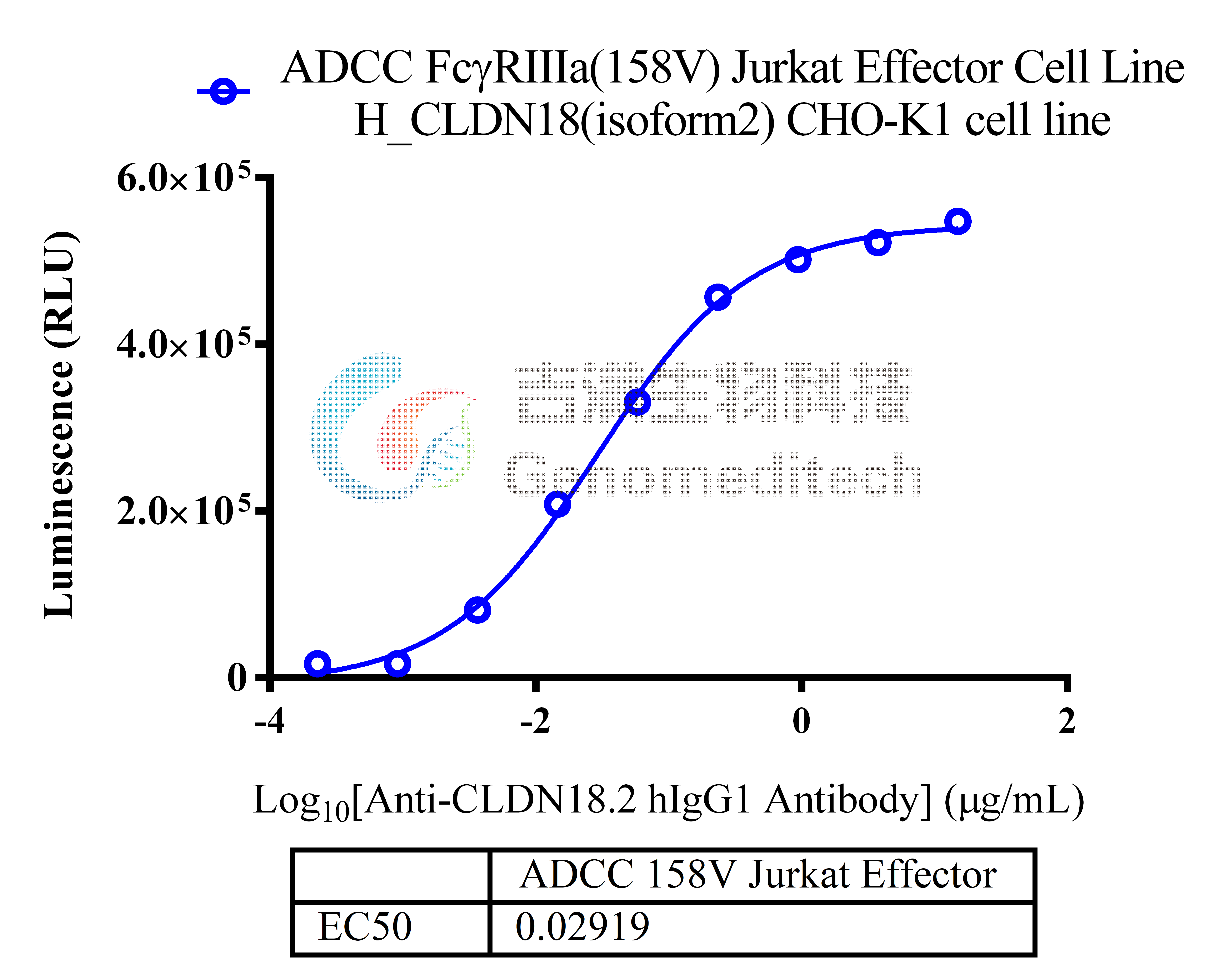
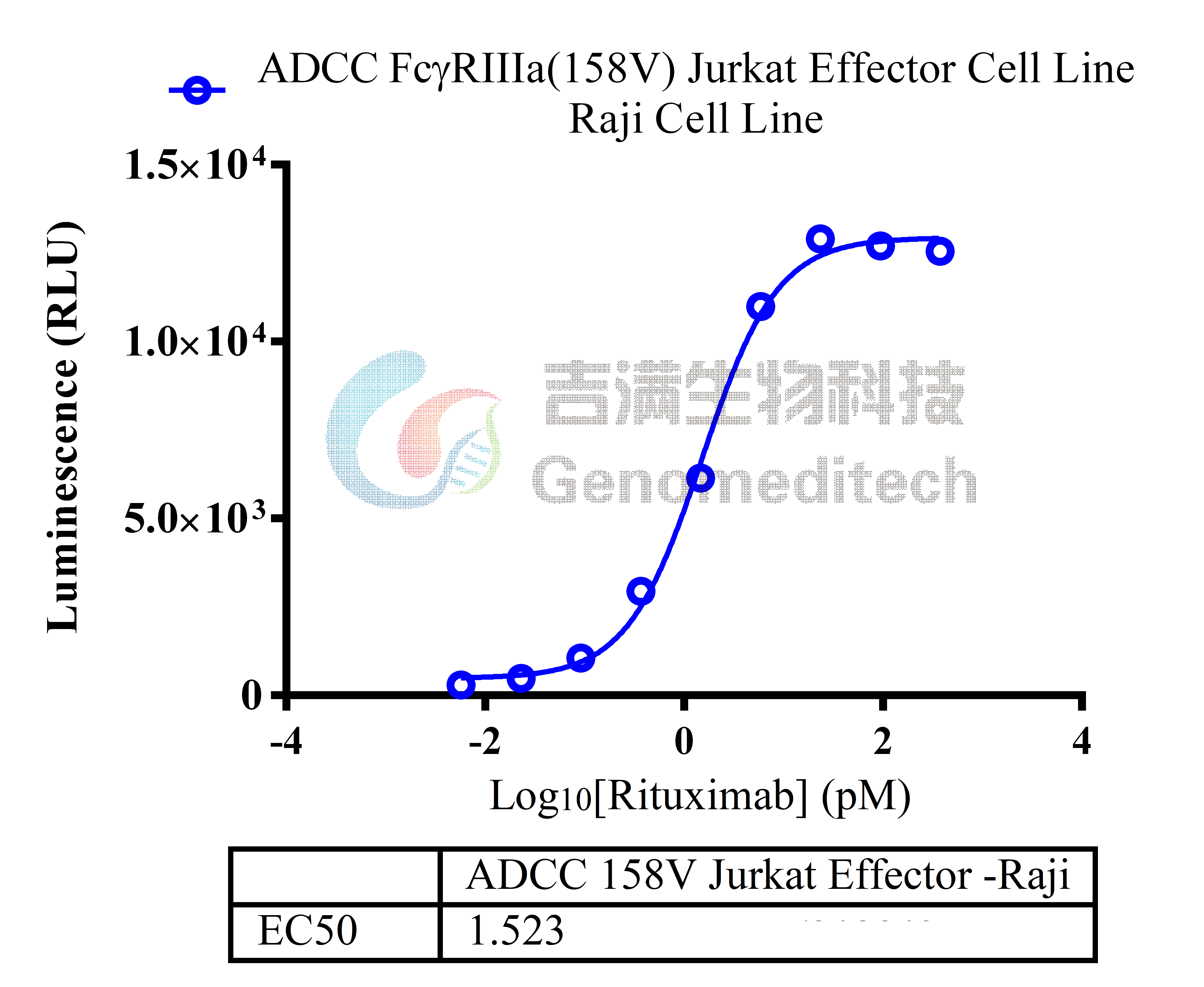
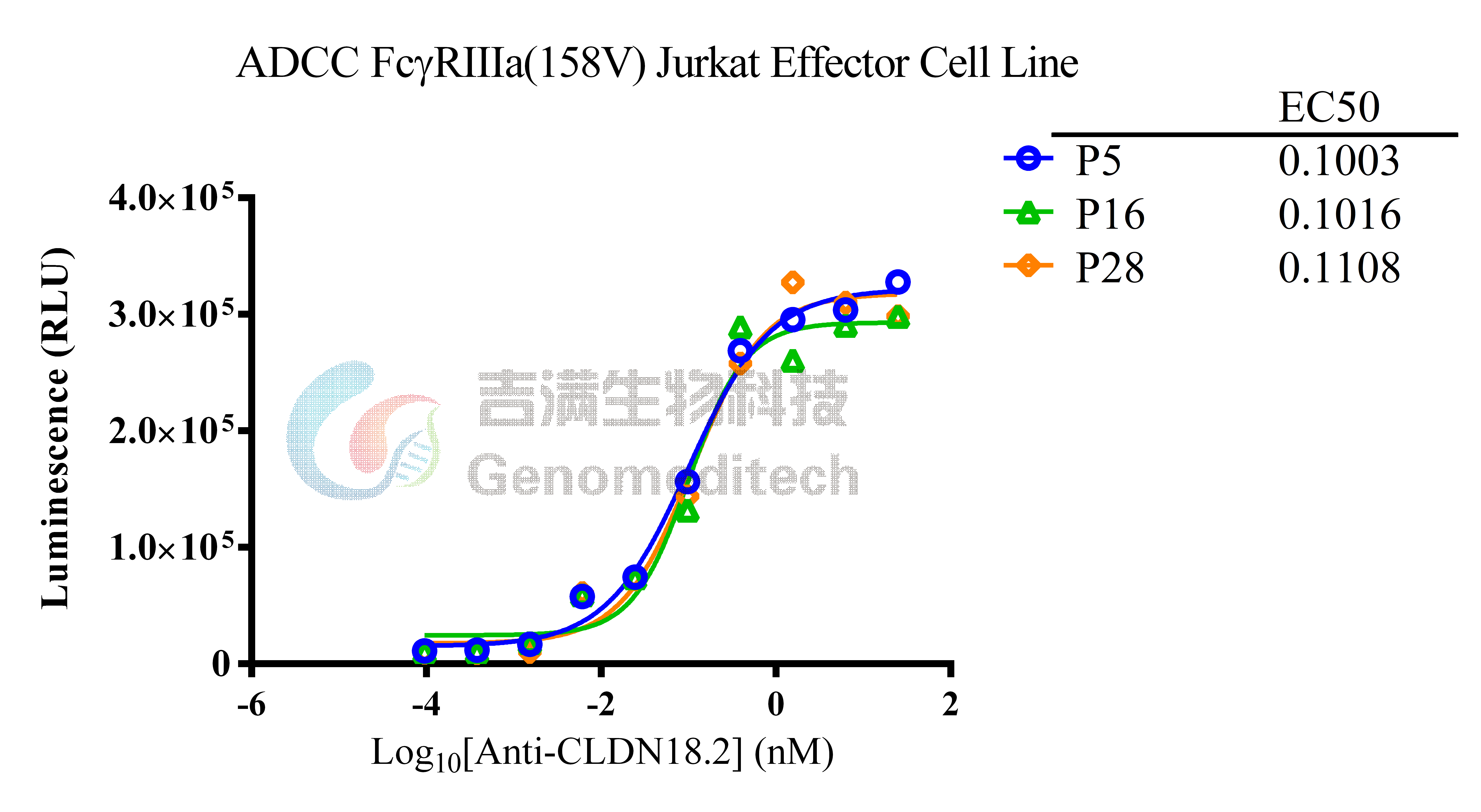
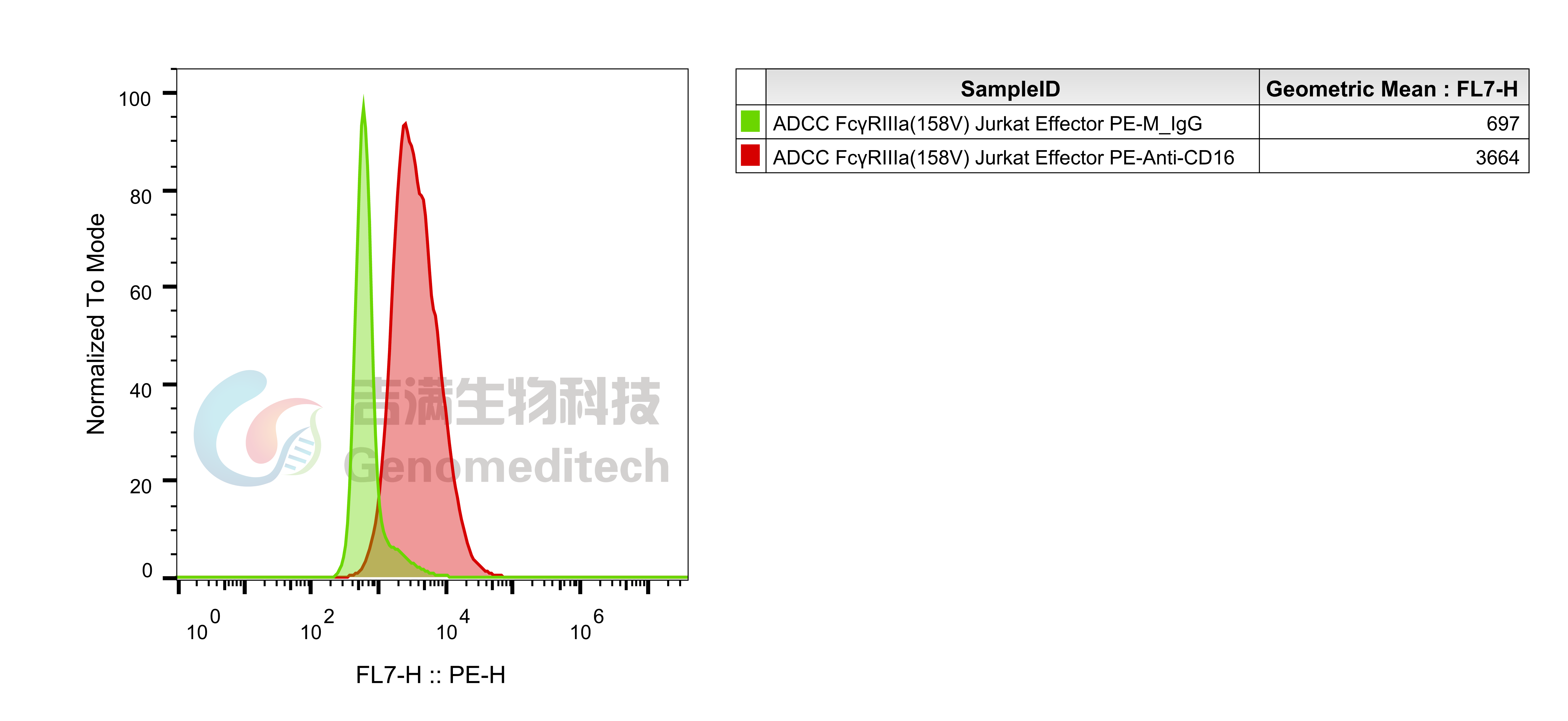
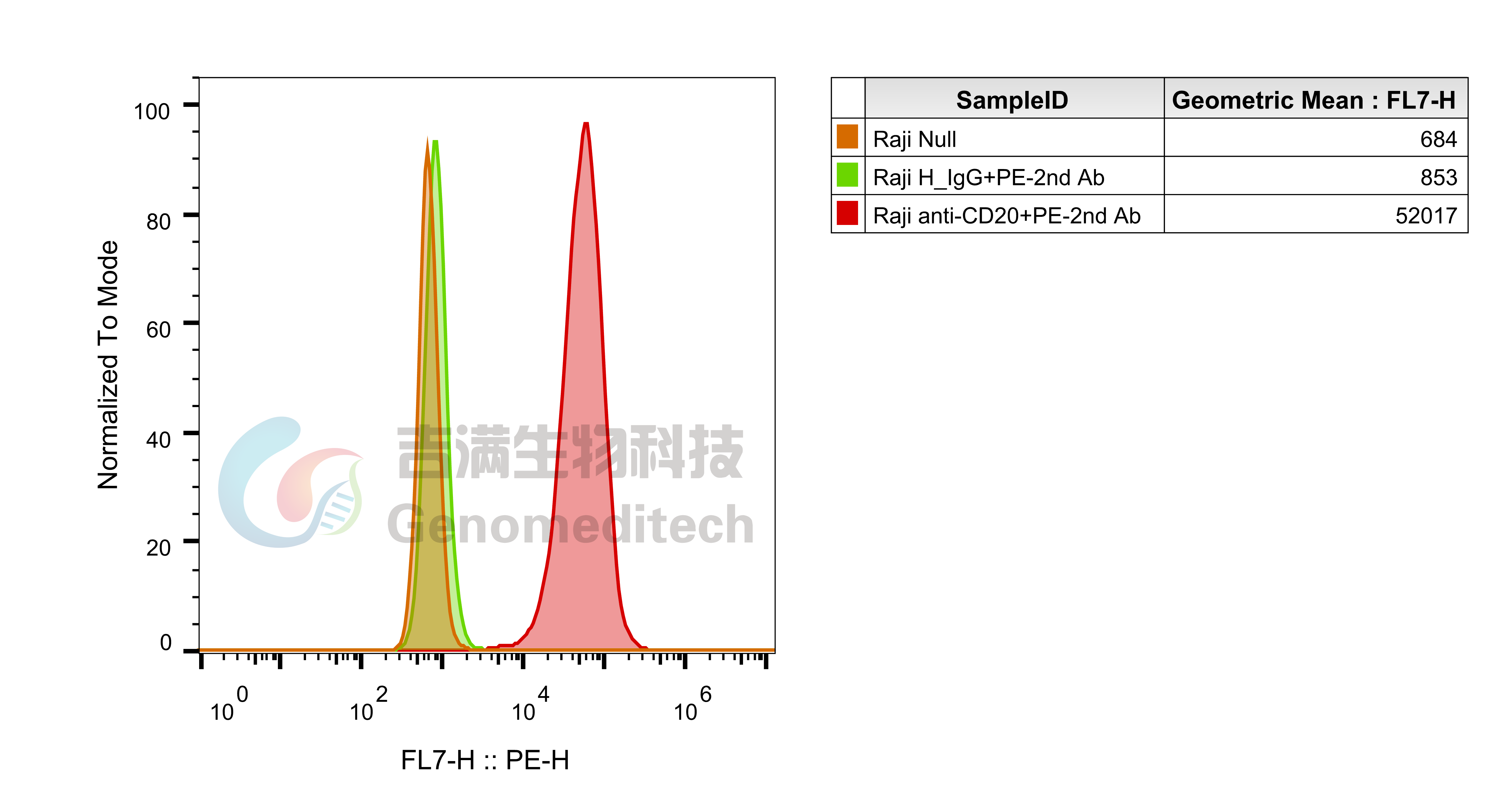
| Cat. No: GM-C05619 Product: ADCC FcγRIIIa(158V) Jurkat Effector Cell Line ADCC, or antibody-dependent cell-mediated cytotoxicity, refers to the process by which immune cells expressing Fc receptors directly kill target cells that specifically bind to antibodies through recognition of the Fc region of the antibodies. Nowadays, the mechanism of ADCC is used to detect and evaluate the efficacy of antibodies or target cells. Antibodies bind to target antigens on the cell surface. If the Fc region of the antibody simultaneously binds to the FcγRIIIa receptor on the surface of effector cells (primarily natural killer cells), the two types of cells undergo multiple cross-linking, leading to the activation of the ADCC signaling pathway. The 158V variant is a polymorphism where valine (V) replaces phenylalanine (F) at position 158, and the 158V mutation exhibits high affinity. ADCC FcγRIIIa(158V) Jurkat Effector Cell Line is a clonal stable Jurkat cell line constructed using lentiviral technology, constitutive expression of the FcγRIIIa(158V) gene, along with signal-dependent expression of a luciferase reporter gene. When IgG binds to target cells and effector cells, it leads to the expression of luciferase, which can be used to evaluate the biological activity of antibodies in the mechanism of ADCC. | 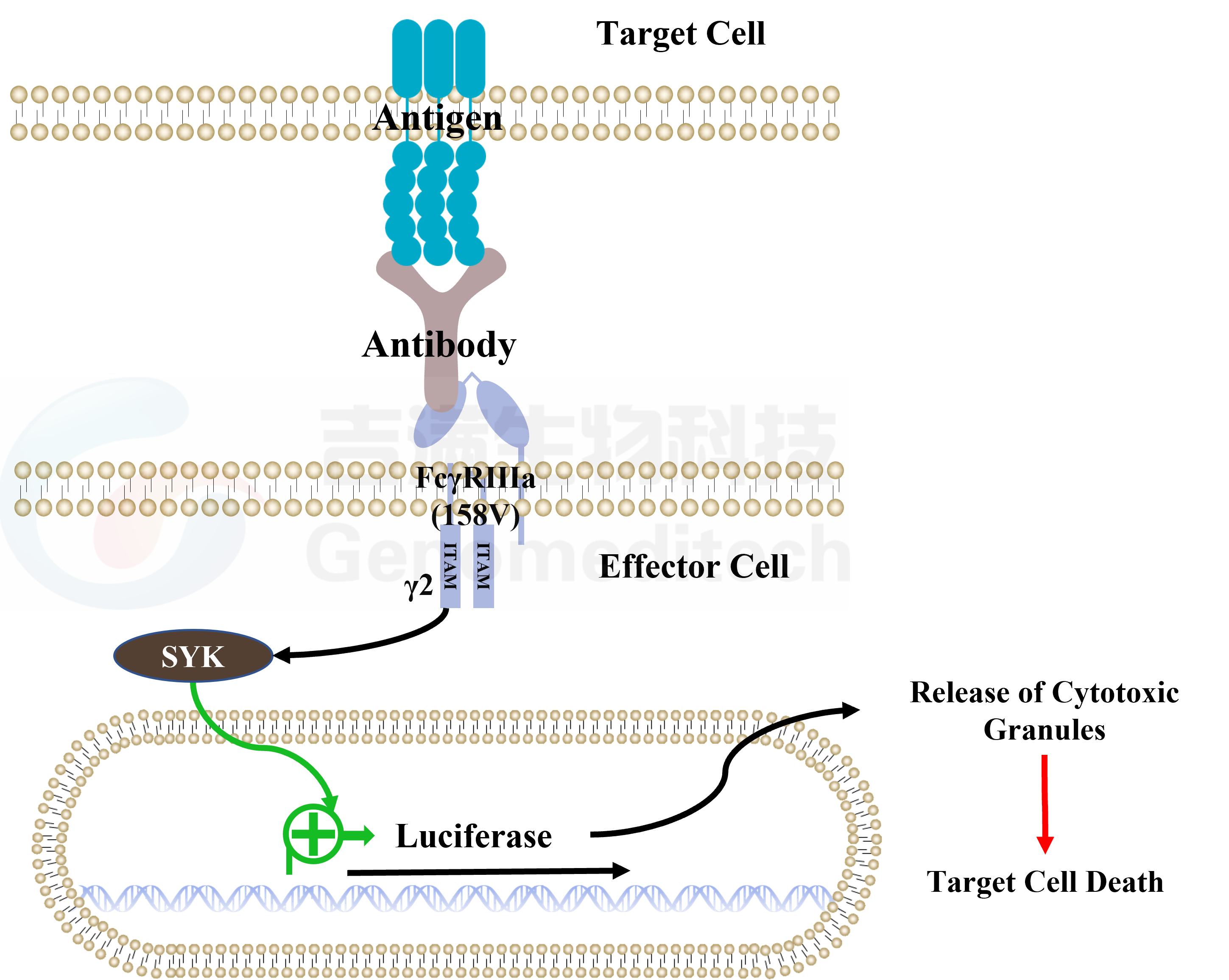 |